Part III of the series: The future of flexible manufacturing
Good process design can maximize efficiency and minimize operational interference in multi-product and multi-phase biomanufacturing facilities. Learn more about process design and two specific segregation strategies that can improve your biomanufacturing.
What process and facility design features permit the rapid changeover from one manufacturing product or process to another? How does facility design minimize operational interference between processes in various stages of the manufacturing lifecycle? By answering these two questions, we can address key design challenges when developing solutions for biomanufacturing multiple products in different product development phases.
Process design and LOUs
Optimized facility design starts with logical and efficient process design. In order to minimize operational risk associated with manufacturing multiple biological products in the same facility, you must have a well-defined process and understanding of the unit operations.
Driven by a design approach focused on the manufacturing enterprise, the key is to define the elements of the enterprise such that operational control strategy is addressed early and that the balance of efficiency and flexibility in the manufacturing operations is met. The three elements of the enterprise must be integrated, the unit operations defined, and the facility attributes coordinated to support a well-defined segregation strategy and operational approach.
Process design focus should be on Logical Operating Units (LOUs). When defining LOUs, it is helpful to begin with three questions:
- Do you understand the process?
- What are the process state and requirements?
- Is the process adequately closed?
Understanding the process definition requires a detailed review of the Process Flow Diagrams (PFDs) and identification of the key LOUs. The focus must be on process transfers in and out of the system and the impact to product protection and process changeover. Under a normal manufacturing schedule, process operations will be in various stages from set-up to operation and shutdown. The goal is to prevent any “train wrecks” that might threaten ongoing operations and put the product at risk.
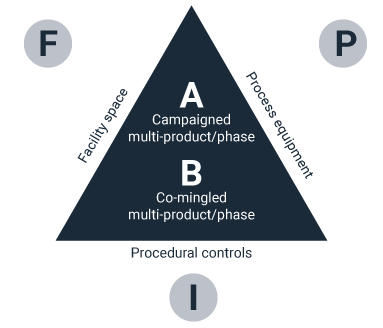
Manufacturing risks increase significantly when the different processes go through their operational cycles of setup, operation, and clean-up for each LOU. If the processes comingle, then all the operational cycles of the different units must be carefully coordinated so that there is no perception of potential cross-contamination.
For example, clean-up of one process cannot occur while another process is being set up. When an LOU for any process fails, it may affect the operational cycles of all the other processes within the room. It is conceivable that a major failure of a single LOU could result in the loss of all the commingled products being manufactured within the same space. So how do you solve this problem?
Segregation strategies
The optimized facility layout approach should support high throughput of multi-products at varying scales. It should also support optimized flow of materials, equipment and waste while facilitating rapid change-over in a matter of days. There are two general approaches in the industry today around segregation strategy: co-mingled ballrooms and segregated matrix-type facility layouts.
The optimised facility layout approach should support high throughput of multi-products at varying scales. It should also support optimised flow of materials, equipment and waste while facilitating rapid changeover in a matter of days.
The ballroom approach
The comingled ballroom approach relies on the ability of the design to validate system closure for all LOUs and define very clear operational controls for every step.
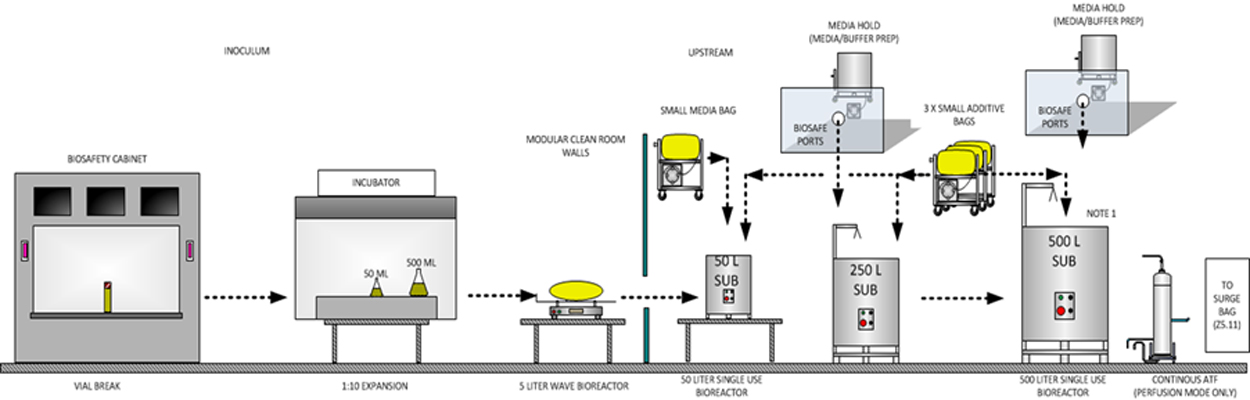
The assumptions for such a layout as the figure above include:
- Controlled not classified (CNC) classification for the main hall and final purification areas
- Closed systems for a single-use system (SUS)
- Shared equipment for products in media and buffer prep, harvest and clarification
- Concurrent manufacturing
The advantages of the ballroom approach include:
- Open architecture, reduced manufacturing footprint and lower facility construction cost
- Reduced HVAC operational costs, up to 33% reduction in air supply[i]
- Reduced air locking strategy
With the ballroom approach comes certain risks, including the aforementioned “train wreck” scenario in the event of closed system failure, increased complexity of validation activities and likely increased regulatory scrutiny.
The matrix approach
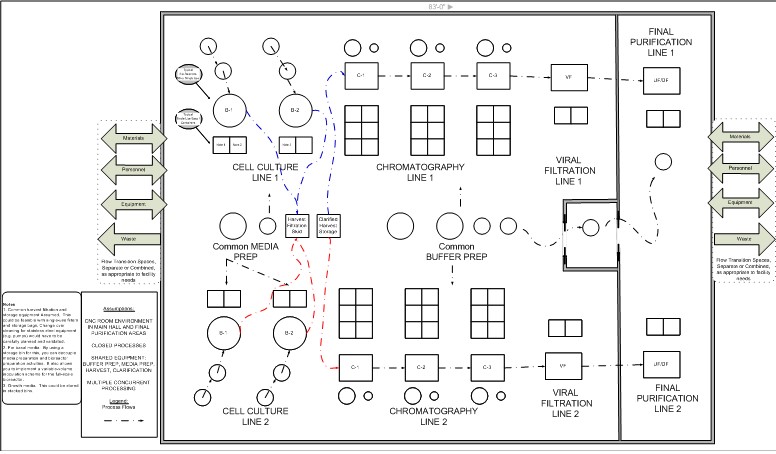
In the matrix layout below [ii], LOUs for a process are located in independent, segregated spaces that are operational in a defined sequence for a campaign when needed. For example, the inoculum area and cell culture can begin without the downstream processes in the facility. As the inoculum process is completed, it is possible to install, prepare and use the single-use bioreactor (SUB) only when needed, and the same goes for the downstream processes.
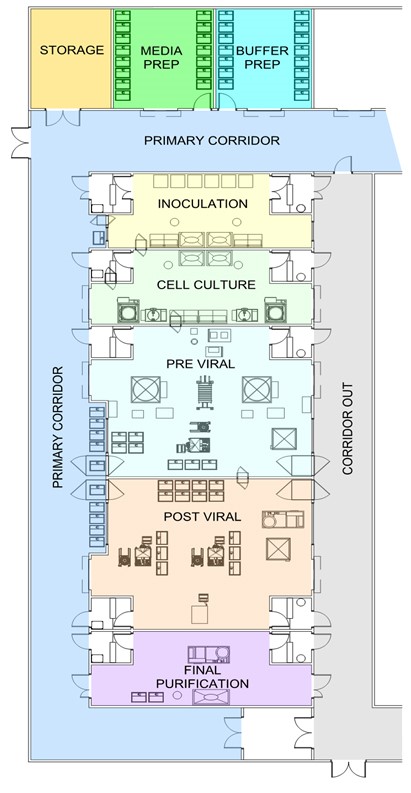
For short manufacturing campaigns, the inoculum and SUBs can be removed as their operation is completed. When they are removed, the operating space they used could be redeployed to another product or the space used for another type of LOU for another process. In high-utilization facilities, it is possible to launch many short campaigns without risking other products.
Matrix designs with unidirectional flows can be modified by minor construction within specific suites through, for example, outgoing waste corridors by carefully timing ongoing outflows from existing production operations with the construction activities. Conventional designs usually are not expandable without the risk of affecting ongoing production. Typically, these designs expand by building additional large suites. Minor construction projects are difficult to complete when ongoing production occurs simultaneously with manufacturing.
Due to its segregation approach, a matrix layout provides increased flexibility, but at a higher cost for architecture and operations. Risk is mitigated since the LOUs and products are segregated within the room, physically segregated from all the other products and processes within the facility.
Through a successfully executed risk analysis, it can be shown that risks increase significantly when the different processes go through their operational cycles of setup, operation and clean-up for each LOU. If the processes are commingled, all of the operational cycles of the different units must be carefully coordinated so that there is no perception of potential cross-contamination. For example, clean-up of one process cannot occur at the same time, as another process is being set up. When a failure of any UO for any process does occur, it may affect the operational cycles of all the other processes within the room. It is conceivable that a major failure of a single UO would result in the loss of all the commingled products being manufactured within the same space.
While it is no easy task to select a segregation strategy, it is a fruitful exercise. Whether your facility will benefit from the ballroom or the matrix layout, both strategies offer a number of advantages and occasional potential risks.
[i] Cochet, O., Pierre Fabre’s New cGMP Clinical Facility for MAbs; a Highly Flexible Cost Effective and Sustainable Design, presented at the ISPE Biotechnology EU Conference, Strasbourg, France, 13-14 November 2013
[ii] “Optimizing the Operational Flexibility and Efficiency of Manufacturing Facilities Required for Launching New Biopharmaceutical Products”, Witcher, Mark and Harry Silver, NNE