Part IV of the series: The future of flexible manufacturing
You have a facility that can handle a wide range of process scales. But how do you ensure that your resources match the scale? Learn which key challenges of strategic manufacturing you must address immediately – from capacity needs to segregation strategy.
How can a facility design efficiently implement various process scales and production requirements, ranging anywhere from 10L to 2000L, so that facility resources match the scale of the process as closely as possible to avoid under-utilisation of resources when smaller scale processes are required?
This is a fundamental question concerning strategic manufacturing and flexibility. Earlier in the series The future of flexible manufacturing, you read about the goal of many Facility of the Future (FoF) efforts (Figure 1). In this type of facility/manufacturing model, the challenge is solving how to support manufacturing capability for a portfolio of products with varying process formats, manufacturing scales and campaign lengths.
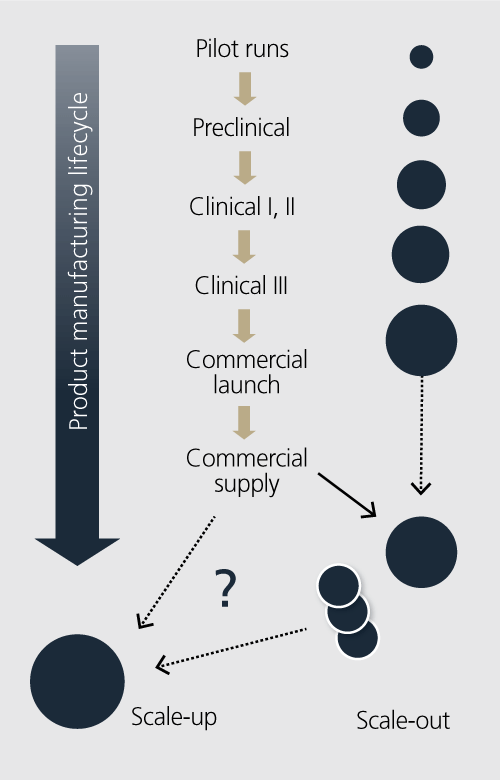
Production scale-up
When attempting to answer this question, you must begin with the production requirements necessary for the product manufacturing lifecycle. Figure 2 presents a simple yet powerful image of one challenge for the manufacturing director – a 1,000-fold increase in manufacturing capability is common. The capacity required in each development phase may increase dramatically based on patient population, dosing requirements and the overall yield of the process.
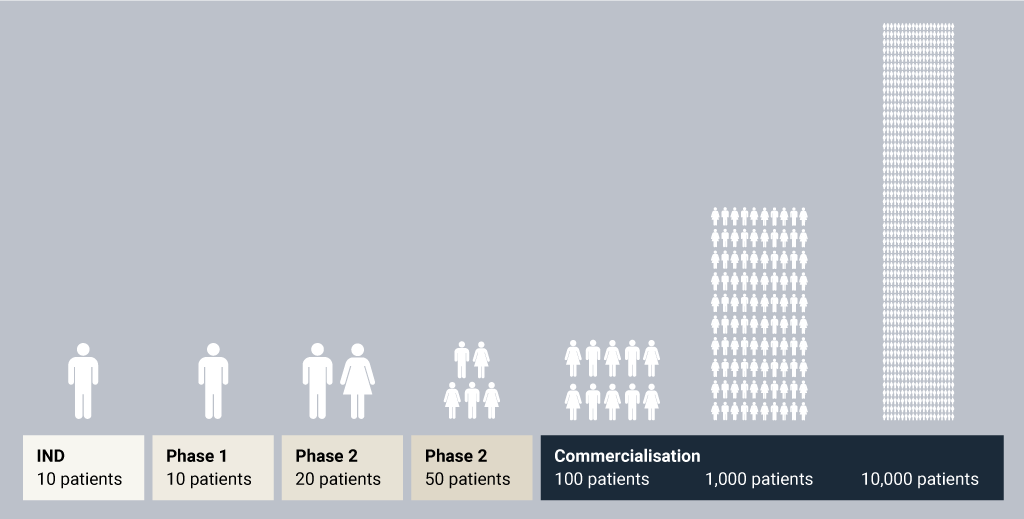
Couple this with timing product campaigns during a production year, and the solving of the scale-up/scale-out problem becomes very important. The goal is for the solution asset to run a wide range of process scales (5L – 2,000L) and to implement hybrid (single-use system and hybrid) with minimal (optimized) resources (Figure 3). This may also include the ability to run a variety of operational platforms including batch, semi-continuous and continuous process platforms.
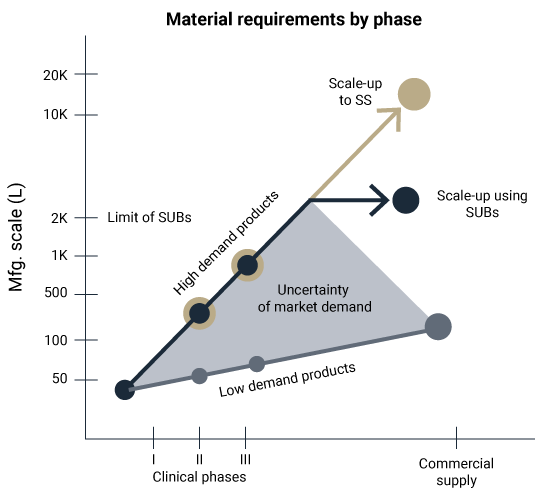
Solving scale-up challenges
In recent years, the major engineering complexities around scale-up that have challenged companies (particularly in fed-batch systems) are magnified when developing a truly flexible, multi-phase, multi-product manufacturing solution. Automated, closed and scalable processes will be the only option afforded in the next FoF in terms of business investment. So how do you address these various challenges?
The challenge: Product/process definition
In early stage clinical manufacturing, an adequate understanding of the process or the critical process parameters (CPPs) required for optimal performance, scalability and robustness may not be defined. Moving from a single-use solution to a stainless-based solution may require additional development activities. Moving to larger scale applications could result in technology changes such as centrifugation in lieu of depth filtration or changes in resin design. In addition, defining control strategy is critical to the overall manufacturing philosophy.
The challenge: Capacity needs
How do you optimize capacity needs when moving from clinical supplies to commercial launch? An analysis of the organization’s product pipeline will give some indication of the distribution of candidate products across the clinical phases. This wide range in capacity not only affects physical manufacturing equipment platforms for upstream and downstream activities, but also impacts critical and support utilities and the management of resource and infrastructure (control strategy) attributes. One example is in the manufacture/delivery of the wide range of buffer chemistries for purification, and determining if the implementation of in-line dilution/conditioning systems is optimal for addressing buffer volumes.
The challenge: Segregation strategy
What is the proper level of applied “control” for commercial manufacturing that also supports early-stage clinical manufacturing? An interesting challenge, especially when you must support a portfolio of products across a wide variety of process formats and campaign lengths. Not all processes are created equal; early stage development-based processes will not have a high level of system closure defined and therefore require more controlled environments for product protection and GMP compliance.
Control strategy is based on knowledge of the clinical performance of the product, its quality characteristics (CQAs and material attributes) and an understanding of the related manufacturing process (CPPs). It is an important vehicle for robust manufacturing, it must be established during development and it can include advanced process analytical technology (PAT) controls to ensure the product is manufactured to meet the expectations as defined in the quality targeted product profile (QTPP). During the development phase, different scales might be involved, but the final control strategy must be implemented into the manufacturing facility and integrated with other controls to support the complete range of manufacturing scale.
The challenge: Technology
How can technology alternatives be used to address scale-up issues in a flexible facility solution? For many biopharmaceutical products in the pipeline today, lower production volumes are the result of improvements in process yields or a focus on personalized medicine-based therapies. However, there remains a need to be able to address scale-up requirements for drug substances, particularly for robust, well-characterized monoclonal antibody (mAB) processes that are traditionally fed-batch. Continuous manufacturing has the potential to allow the production of large product from smaller, flexible manufacturing footprints, facilitating a scale-out strategy for commercial launch and supply. Here, continuous manufacturing facility design represents a scale-out solution versus the fed-batch facility design based on scale-up.




