Part I of the series: The future of flexible manufacturing
Get flexible or fade out. When it comes to the complex journey of facility design, vision-drivers for flexibility must be determined early on. But how do you define flexibility, agility, simplicity and innovation?
If you consider the future of biomanufacturing, one of the vision-drivers is to develop flexible manufacturing assets that provide a high level of value and innovation. These facilities have a foundation based on one or more of the following:
- Multi-product
- Multi-phase
- Multi-scale
- Agile manufacturing platforms
- Simplified interfaces
If these operational attributes are the target goals of a design program, what then should be the key drivers of the design effort? While there may be any number of drivers identified from both a business and technical perspective, five come to mind.
1. Process scale
The manufacturing output:
- Drives decisions around equipment platforms,
- Influences the physical size and adjacencies in facility architecture
- Motivates the selection of technology platforms
- Affects the overall segregation strategy of the layout
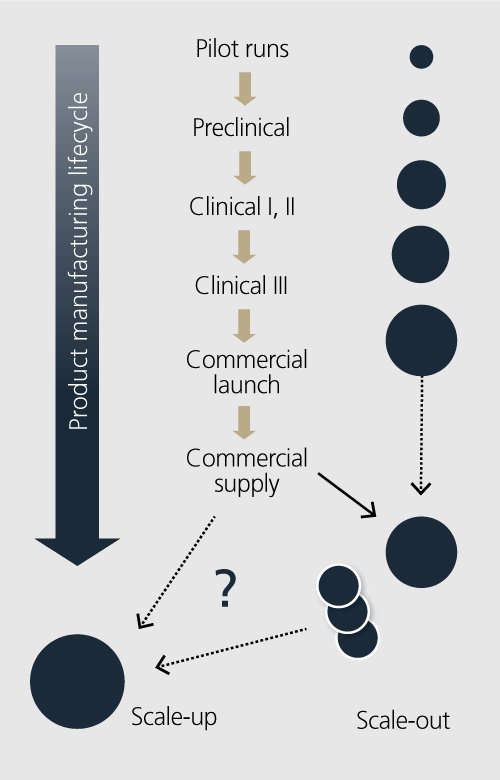
If a company envisions a strategic approach to meeting the product manufacturing lifecycle, and envisions a facility that can manufacture clinical, launch and commercial materials, then the issue of scale becomes a key driver. This manufacturing lifecycle includes large-scale pilot runs and pre-clinical materials or even commercial launch quantities. The option of scale-up or scale-out then becomes a driver for how to design the asset.
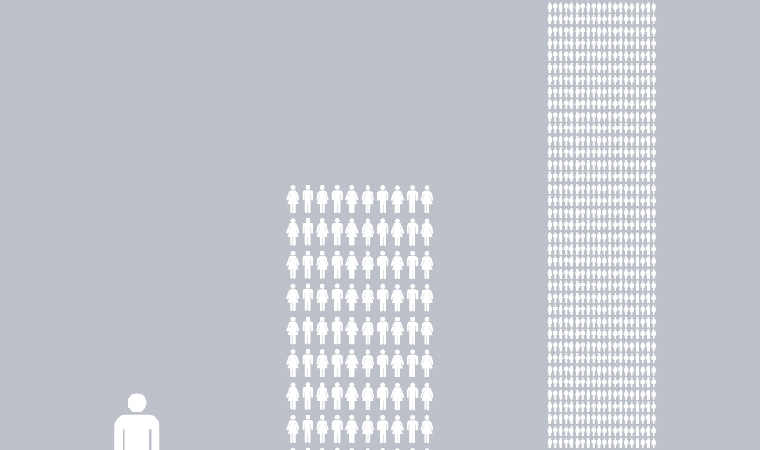
A truly flexible manufacturing facility is able to manufacture a wide range of process scales – from early phase scales at 5 to 10 L all the way up to commercial launch scale of 2000 L.
Keep in mind that the scale of production influences the overall production cost of the enterprise. Maximizing this efficiency of scale must also be a key aspect of the design approach.
2. Rapid changeover is essential
If the manufacturing ambition is to provide efficient multi-product capabilities, then efficiency in changeover between product runs is essential. Again, segregation strategy and technology platform selection must be synchronized.
Whether the platform utilizes traditional stainless steel, single-use or a hybrid of the two, the ability to move rapidly from product to product is vital. This ability includes ensuring that the design solution addresses manufacturing risks such as change-parts, cleaning and system closure.
Often, process design builds around single-use systems (SUS) and components. This introduces a key aspect of changeover efficiency. While SUS is labour-intensive, decisions around pre-packaged or fabricated components can improve changeover timing and durations. As an example, if a third-party supplier prefabricates and tests all tube sets, it allows for tube set inventory and designates the supplier as part of the Supply Chain control from a quality and compliance perspective. Other important changeover attributes include:
- Staging logistics for portable skids/equipment
- Routing access for tube sets
- Waste handling/removal
- Ease of Component Identification via RFID
3. The process and human interface needs to be simple
Flexibility and agility have a common attribute – simplicity. Flexibility requires high levels of efficiency and rapid changeover - so the simpler the human-machine interfaces are, the higher likelihood of success. These interfaces include equipment access and transfer, system connections and de-connections, process control and record generation.
Simplicity in flexible manufacturing facilities is critical. How easy is it to set-up multiple processes within a given suite configuration? When using SUS components, is identification easy for the operator? Is the movement of a skid from one location to another easily accomplished? At every point where a human encounters the process, it is imperative to evaluate the interface for simplicity against available technology.
4. Expandability for maximum efficiency
Flexibility often requires providing future capabilities to expand operations for a number of reasons – new or expanding markets, improved or new technologies and a growing product demand. This expansion must account for minimal disruption of ongoing operations, maximum efficiency to preserve cost-of-goods metrics, technology optimization and schedule improving to implement the expansion solution.
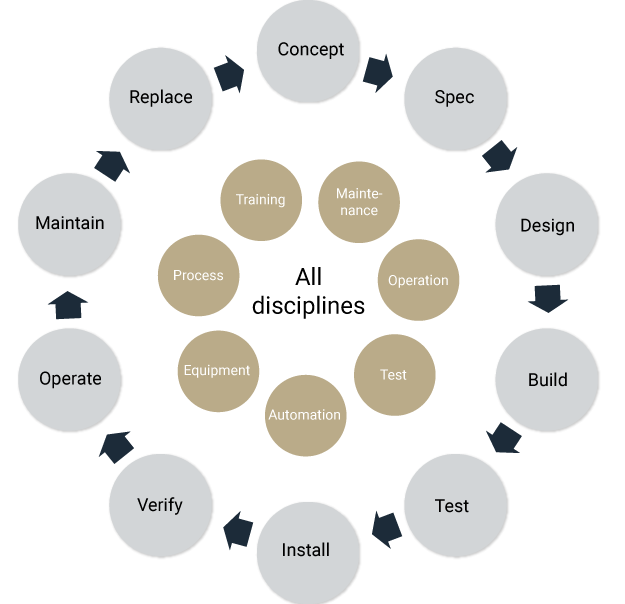
A modular design approach, in which flexibility drives expansion throughout the complete project lifecycle and all disciplines, is an efficient approach. A facility can achieve a high degree of flexibility by using “standardized” modules. Standard modules can reduce both cost and time and can function cross-disciplinarily with clear interface designation. This approach might include:
- Modular product design (standard components)
- Modular processes (standard unit operations)
- Modular equipment (standard process units)
- Modular control systems (compound recipes)
- Modular procedures (compound documents)
- Modular training programs (training modules)
5. Control strategy is the backbone of GMP
All the other drivers identified have a common link – the dependence on a well-defined operational control strategy. Control is the backbone of good manufacturing practices (GMPs). The control strategy is an important vehicle for robust manufacturing. It must embrace methods, such as Process Analytical Technology (PAT), that ensure products meet the expectations defined in the Quality Target Product Profile (QTPP).
The recognition and establishment of manufacturing as an enterprise model is important. To achieve the GMP goals of establishing control, providing proof of control and ensuring a means of validating a return to the controlled state in the event of an issue, effective design solutions for the process, facility, and infrastructure elements of the manufacturing enterprise must be defined early in the project lifecycle.
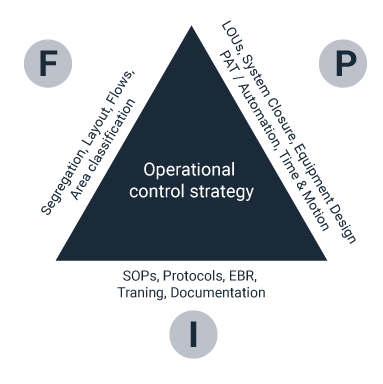
In addition, these elements must integrate with each other to support the robust manufacturing target. This could involve defining trade-offs early in the conceptual design process between each of these elements.
Summary
Organizations view flexibility as something facilitated by both business and technology-focused drivers. Speed, cost and compliance are attributes that affect the finished solution.
Addressing the above will not solve all issues. However, getting a clear solution in place to address them early in a project will lead to a higher probability of reducing cost of goods and ownership, minimizing risk and supporting business-focused design solutions.