While other manufacturing industries discuss Industry 4.0 and Big Data, pharma manufacturers are securing the integrity of their regulatory records. Data integrity compliance tends to be reactive and many see data as a liability. Yet putting data in play increases agility and efficiency - and can be a great opportunity for digital transformation.
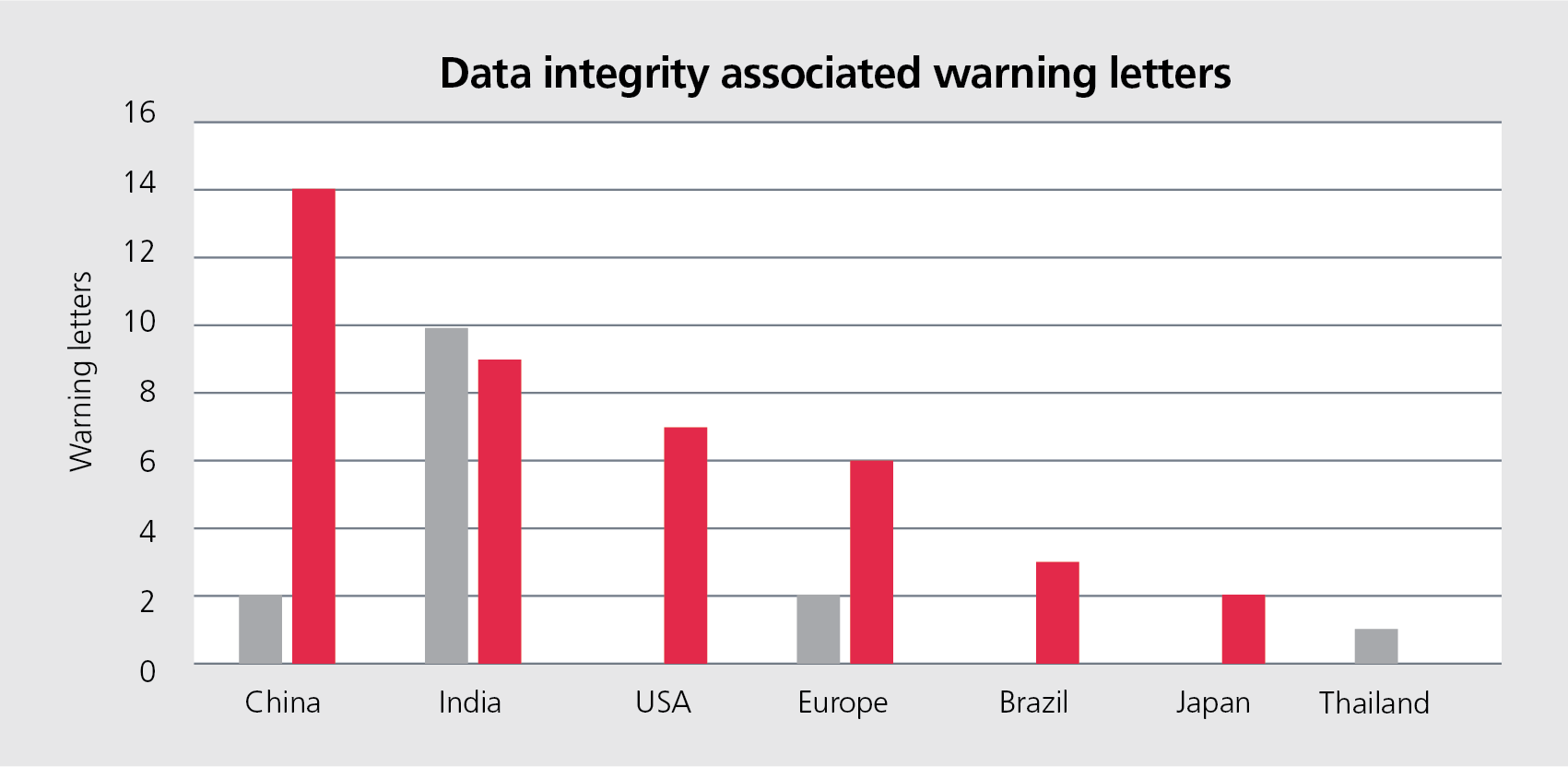
Warning letters related to data integrity have increased in recent years. As you can see in the graph below, with the exception of India and Thailand, the amount of warning letters rises significantly from 2015 to 2016.
Ultimately, regulations related to data integrity can either hinder or propel us forward. Somehow we need to marry new regulations with the changes we want to see in pharma manufacturing.
Data integrity in pharma manufacturing
This may be the most thorough data mapping exercise in the history of the industry, and often results in a significant tightening of data management activities. Therefore, improving data integrity compliance should align to these overall goals:
- Acknowledge that regulatory records include raw data records.
- Specifically define what your records are.
A new approach to data: Putting processes first, and systems last
The traditional approach to compliance is based on the qualification of systems. Typically, these requirements are stated in the User Requirement Specifications (URS). Data associated with individual systems are then defined.
Yet with this approach the overall context is forgotten and data is not properly integrated into the process. In the future, we need to first define processes, then define data, and then specify data flows across the IT architecture. In doing so, we properly focus on processes and make sure that a facility matures in relation to recorded information.
Ultimately, processes and records (and data generally) must be the starting point for engineering and qualification.
In the future we need to first define processes, then data, and then specify data flows across the IT architecture. In doing so we can properly focus on processes and ensure that a facility matures in relation to recorded information.
Incorporating a quality culture in pharma manufacturing
Pharma manufacturers traditionally claimed they had difficulties adopting manufacturing excellence because of regulatory requirements. Since then, regulators have adopted a risk based quality paradigm that offer manufacturers tools such as risk assessments and control strategies for managing product quality. These tools enable continuous improvements. What remains is a change in culture: building the necessary motivation and competences.
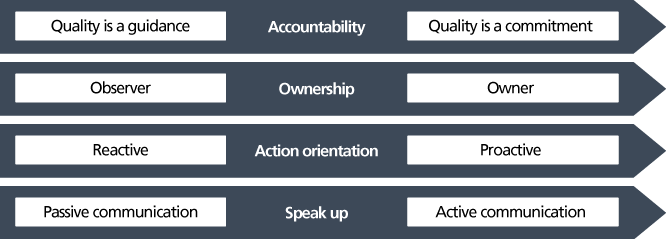
To truly embed quality into your organizational psyche, you have to provide more than just guidance that people observe and react to. Instead, we need to shift towards a proactive dedication to quality that people feel they have ownership over. This can include tying quality to core values, distributing a code of ethics, and providing a transparent and open environment for reporting problems.
Data integrity and modernizing quality management go hand in hand. For example, data integrity will enforce GMP further in Automation and IT. By incorporating quality culture principles, you ensure that the entirety of your organization is on board for current challenges and future improvements.
Digital transformation
So how can this lead to a digital transformation? You can usually correct gaps in data integrity by documenting and controlling the IT systems you already have. However, from time to time, you may want or need to introduce new systems.
This is a delicate matter, as immediate corrections are justified by local business cases. The result is alternative solutions to those recommended by digital transformation strategies.
Going digital is an enterprise wide effort
As an example, an important enabler for data integrity would be proper IT infrastructure services for user access management and time synchronization. Therefore a proper IT infrastructure program will improve data integrity and automate IT support processes at the same time.
Without a corporate roadmap based on an operating model and enterprise architecture local organizations can take matters into their own hands. This means more variation in operating practice and an increasingly complex system landscape.
To sum up, with proper central support, data integrity can be the stepping stone for digital transformation. By developing an operating model and an enterprise architecture, you clearly define overall corporate responsibilities, allowing local organizations to use centralized solutions. And by putting data first, manufacturers can properly integrate data into all their processes.
Sources: