Though utilized by many players in the manufacturing world, QbD principles are still not the standard for all manufacturers’ pharmaceutical facility designs. Why should the pharma industry take QbD approaches more seriously? Spoiler alert: it involves the patients.
Quality by Design (QbD) is a science and risk-based approach, defined by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) quality guidelines Q8, 9, 10, 11 and soon 12.
Many pharmaceutical manufacturers implement these principles, but there is still miles to go before every manufacturer embraces them. However, this is gradually changing as the pharma industry draws inspiration from many other industries where QbD has been the common practise for decades.
High efficiency, minimal rejects
As a manufacturer you naturally aim for increased efficiency and minimal number of rejects, whether it be intermediate or finished products. This is achievable by building quality into your products and processes and by applying a rigorous scientific and risk-based approach throughout the product lifecycle. This integration begins as early as development, continuing through to manufacturing and discontinuation.
It is not difficult to integrate the QbD approach in all phases of your product lifecycle. In fact, QbD principles link seamlessly to Six Sigma principles. When combined, these standards improve process robustness. They reduce process variability, waste and rejects of your existing processes, and establish the appropriate controls.
Following an analytical approach to production processes and focusing on variance gives you an extensive process understanding like never before. This knowledge is priceless, both when registering applications with regulatory authorities such as the US FDA and when dealing with unexpected production deviations.
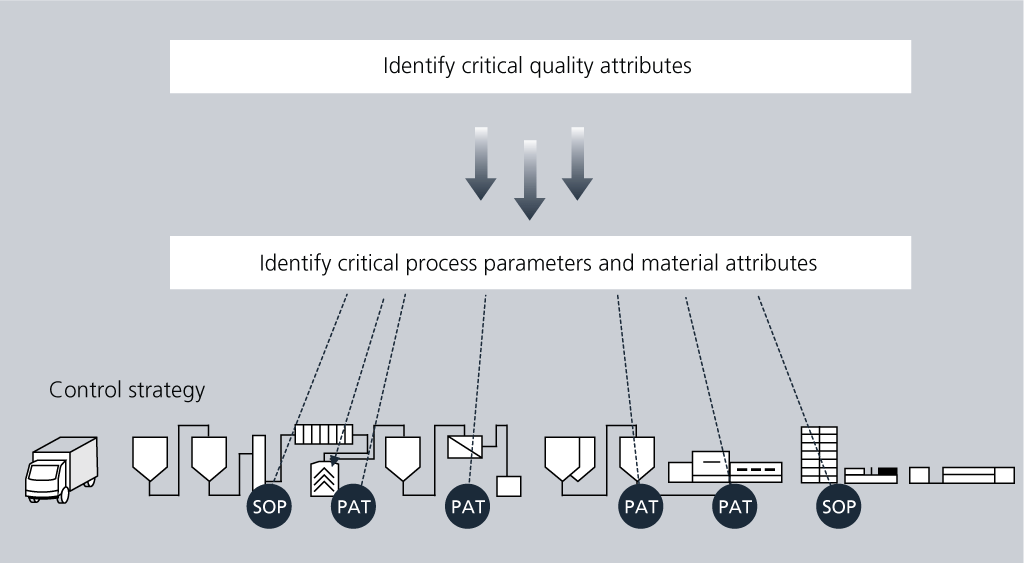
The control strategy reflects the current level of process understanding, i.e. the understanding of the CQAs and how the CPPs and material attributes influences these and should be controlled. The control strategy can include PAT-based controls, IPC, SOPs, specifications, etc.
Satisfying the regulatory authorities
Regulatory authorities encourage the QbD approach and acknowledge the value of an increased level of process understanding. At the end of the day, this motivates better and more robust products on the market, which minimizes the risk of drug shortage.
In an attempt to force more process understanding into the medical industry, authorities in many countries have begun requesting extensive information about product quality attributes, production processes and related process parameters in connection with product registration and approval.
Quality by Design is intended for implementation during drug development, but the principles behind it are applicable and valuable in a number of other contexts. A science and risk-based approach to process validation can ensure the process is aligned with future regulatory guidelines and help keep patient risk at the top of the agenda. And quality design and process optimization is only useful when it helps the true end-users – the patients.