Pipelines are increasingly diverse and volume demands uncertain. How can biotech players stay afloat in this changeable manufacturing landscape?
Biotech is moving beyond the traditional “one drug, one protein, one disease” paradigm to pipelines with multiple sophisticated new drug modalities. These include complex versions of traditional protein based molecules – such as enzymes, hormones, monoclonal antibodies and fusion proteins over antibody-drug conjugates (ADCs) – and the quickly evolving area of cell therapy. The ability to gain deep biological insight into complicated diseases and tackle them with sophisticated, specifically engineered drugs will save millions of lives worldwide.
To adapt to this change, biotech manufacturers will increasingly face new business scenarios. As positive as these developments are, they also mean more product variation, uncertainty around capacity and a need to stay on top of different regulatory requirements. In addition, society is changing. People are increasingly focused on environmental sustainability and smart workplace design to improve employee wellbeing.
Taking these factors into consideration, the biotech manufacturing industry must be increasingly flexible to cope with shifting demands. Reacting to these changes by finding the right combination of new technologies and agile manufacturing techniques is key.

The impact of business changes on biotech manufacturers
One scenario is dealing with the urgent need to increase the output of an established facility. This means increased run rates, increased intensity and yield and the need to build additional capacity. Ultimately, a manufacturer would need to find the right technology to up the production capacity in the most cost efficient way.
However, there may at some point be less need for this product, or the need for the product may be delayed. A manufacturer would then need to reduce the run rate and potentially introduce new products to the pipeline. This would increase their focus on flexibility and producing smaller yields.
In a multiproduct scenario, there would be a need to produce additional products and clinical material. To do so a manufacturer would need to think about tech transfer and fast changeover.
Finally, perhaps a manufacturer needs to focus more on health, safety and the environment. They would then need to find ways to reduce environmental impact while improving employee wellbeing without disrupting operations on a large scale.
What’s the right combination? Leveraging technology and agile manufacturing
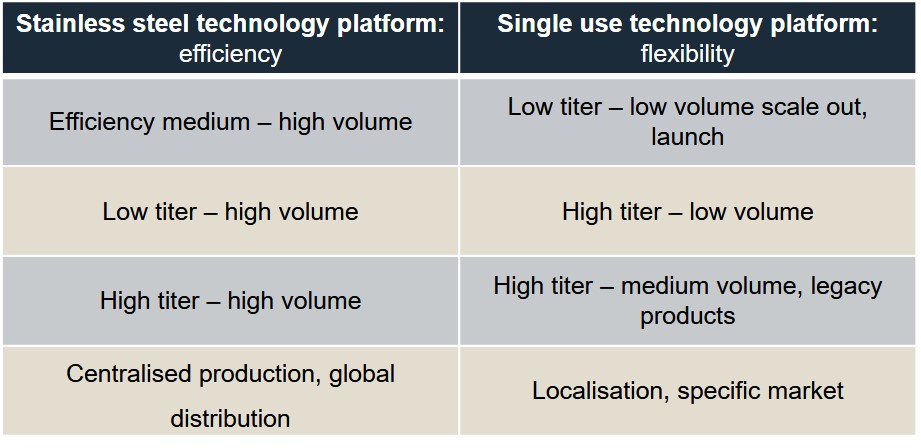
Different facility types, from large-scale stainless steel fed batch to small scale personalized medicine, can leverage different technologies to cope with these changes – including titer increases, single use, continuous and closed processing, process intensification, PAT, digitalization and robotics.
For example, stainless steel technology platforms offer a high volume output and increase efficiency, whereas single-use technology platforms are lower volume, more flexible and can be localized to a specific market.
Utilizing a modular approach increases the flexibility of a building, as well as the execution and operational methods, offering the opportunity to adapt quickly to changing demands. This includes:
- Fast-track project execution
- Modular processes
- Installation-friendly buildings
- Integrated teams
- ASTM-E2500 verification
Ultimately, it is paramount to remember – what may make sense today may become a stumbling block in the future. The key is to investigate a broad array of different scenarios and find the right combination of technologies that address both your current needs and allow you to stay agile and switch up your approach if business needs change.


