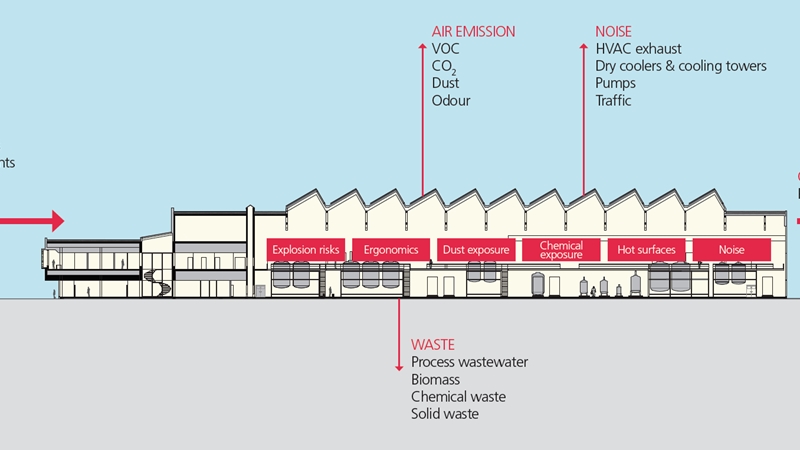
Active pharmaceutical ingredients (APIs) have become stronger and increasingly more active in recent years. The manufacturing of these products – and particularly high active pharmaceutical ingredients (HAPIs) – require high containment facilities in order to:
- Protect the product from contamination
- Avoid cross-contamination between products
- Protect the operators from hazardous products during manufacturing processes
- Protect the environment from harmful agents
Establishing a combined GMP and containment environment is a complex task that relies on a fine balance between various sets of local and global requirements. With our long experience in GMP and containment facility design, NNE can help you achieve the perfect balance, by combining these key competencies:
- In-depth knowledge of cGMP and containment
- Understanding all aspects of pharmaceutical processing, from receiving to dispatch
- Relevant technology expertise within closed process and barrier/isolation technologies
- Risk-based approach to pharmaceutical, biotechnology and laboratory facilities
What is the right level of containment?
Global regulatory authorities such as the European Medicines Agency (EMA) and US Food and Drug Administration (FDA), are not only supporting the high containment trend, but driving it. They call for “containment at source with engineered solutions”. Accordingly, demands have become increasingly direct, restrictive and stringent, requiring manufacturers to implement additional methods, machines and building requirements.
When it comes to handling highly active substances in pharma manufacturing, finding the right level of containment is of paramount importance. NNE can help you evaluate the extent of your containment needs correctly. We will help you interpret the regulatory requirements and put them into the context of your manufacturing needs and set-up. And we will help you identify which issues you need to considered with regard to GMP and in comparison with occupational safety.
The potency level of your products influence a number of aspects. Thus, when doing a safety assessment, we look at what it means for:
- The building
- Ventilation
- Operation
- Laboratories and laboratory testing
- Process and process management
The optimal containment solution looks to the future
Since the activity and potency of substances continues to increase, containment requirements also become progressively stringent. Open production processes, for instance, may not be approved for the production of substances in the future, because the operators will not be sufficiently protected. Thus, your containment concept should ideally consider a higher safety rating than is required for your current product(s), in order allow for future adjustments.
For decades, NNE has helped customers across the world to design new containment facilities, upgrade running production and laboratory facilities and update single process steps to reach a state-of-the-art containment solution, tailored to the customer’s needs. Providing specialized knowledge within containment and cGMP facilities, we can help you:
- Find the right balance between GMP versus containment
- Complete a containment risk assessment
- Reduce cleanroom cost through use of new production technologies (e.g. continuous manufacturing and automated dispensing)
- Plan and design high containment facilities
- Achieve modular GMP and containment facility solutions for R&D, pilot scale to large scale production
- Design airlocks which support containment safety procedures
- Upgrade your existing production environment to be compliant to HSE demands
- Reduce energy costs in your controlled environments
- Ensure that your GMP and containment facilities are operator-friendly and support lean operations
Mitigating the risk in containment projects
Containment projects are complex. Changes or technical errors at a late stage can potentially cause a delay in the start-up of your facility and result in a significant increase of your investment. This calls for a different planning and design approach compared to non-containment facilities to accommodate the demands for a containment facility.
At NNE, we apply our unique containment project knowledge to help you significantly reduce the risk of unforeseen challenges in your containment project. We start every containment project by building a solid understanding of the products and the manufacturing process steps as well as all facility processes and work procedures. As such, our approach to containment projects and containment facility design involves a high degree of front-loading. This also means that we can support you already in the R&D development phase, if required, to ensure safety for your R&D and laboratory staff.
Our front-loaded approach enables us to address issues in the earliest project phases and ensure that initial assessments are developed in close corporation with your HSE (health, safety and environment) officers and user groups and project development/management. The initial project assessment will form a robust and holistic basis for addressing regulatory compliance issues as well as containment design solutions.