Laboratories is an integral part of modern pharma facilities and a part of the pharma value chain. NNE works closely across architectural and engineering disciplines to deliver optimized and efficient quality control and research operations that meet the needs for flexibility, long-term capacity and regulatory compliance. Our specialist skills include:
- Quality control laboratories
- R&D laboratories
- In-vivo facilities
- R&D facilities
- Small-scale production facilities for personalized medicine
- Multipurpose laboratories and vivariums to support multiple products
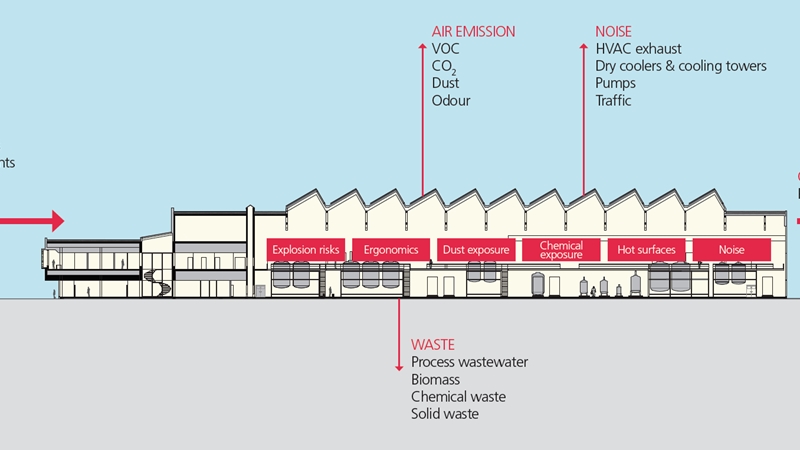
Laboratories are key for pharma and biotech companies, from basic research, through drug development to the final quality control of production batches. Drug development and quality control require laboratories that are in GMP compliance. If the substances include biological agents or genetically modified organisms (GMOs), the laboratories must in addition comply with biocontainment requirements and have a carefully planned and functional design.
Research and development in pharma is under significant pressure. R&D costs and timelines are increasing, resulting in reduced competitiveness. One way to obtain the desired output at the right cost is to improve and optimize laboratory efficiency. NNE offers a number of services to achieve this, ranging from implementing Lean techniques and re-organizing existing facilities to programming and designing new facilities that support the needs of a rapidly changing business.
Our pharmaceutical laboratory planning and design experts come from various backgrounds, e.g. molecular biology, chemistry or architecture. We leverage our varied competencies to help you:
- Minimize investment risk through front-loaded and initial laboratory planning
- Design laboratory facilities that facilitate innovation and productivity
- Reduce operational cost with reduced energy consumption
- Reduce project cost with generic laboratories
- Adhere to bioethical principles in animal research and trial facilities
- Achieve sufficient flexibility and changeability
- Ensure regulatory compliance for GxP, biocontainment and GMO laboratories
- Integrate and optimize operations through technology, laboratory IT and automation
Lab design: Creativity for better results
Modern research is no longer just a matter of creating the ideal laboratory. Today, it requires creating an entire holistic facility with laboratory space, support space, collaborative and social space that provides integrated synergies. At NNE, we focus on establishing complete facilities to match your project mission, business needs and future vision. We firmly believe this to be the catalyst for innovative drug research and development and quality control laboratories.
NNE not only designs laboratory facilities – we facilitate creativity. We work with a wide range of pharmaceutical companies to create the positive and innovative framework they need to foster creativity. The customer's operations, workflows and functionality is the primary driver for how we approach laboratory projects.
Animal facilities built on bioethical principles
Animal facilities play a major role in modern research by ensuring valid research and development results or by delivering your products in full compliance with all regulatory requirements. More and more companies are focusing strongly on compliance with internal standards in bioethics, including animal and employee welfare, when designing animal facilities.
At NNE, we can help you establish cutting-edge research facilities that fulfil the highest ethical standards for animal research without compromising employee or animal safety. We work in close cooperation with you and other stakeholders – such as animal welfare organizations and top level corporate social responsibility (CSR) officers – to establish cutting-edge research facilities that fulfil the highest ethical standards for animal research.