Considering which risk analysis is best for you? When it comes to complex drug development and pharma manufacturing, it pays to prioritize. RAMM combines details with an overview – without slowing you down.
Risk analysis is the foundation of drug development and manufacturing. Much like the multiple products the pharmaceutical world produces, risk analysis tools are equally varied. However, they are often traditionally categorized plainly as simple or detailed, with the former serving as precursors in early development to the detailed tools.This was developed for the batch production system.
However, a continuous manufacturing model requires a different approach. In this article, we consider the risk-based approach used in continuous manufacturing in the car industry.
RAMM - a happy medium between basic a complex tools
In the figure below, RAAM provides a pragmatic compromise where other risk tools might be too simple or complicated, such as a Preliminary Hazard Analysis (PHA) or Mode Effects and Criticality Analysis (MECA).

The most complex part of this pathway is understanding which raw materials and process parameters impact the critical quality attributes (CQAs). This is because this understanding can be used to develop more robust processes or add appropriate control measures. It is therefore a great starting point for a six-stage risk management system for continuous processes.
Step 1 – Team formation
First, you need to find the right team for your RAMM workshop sessions. In later stage development – the more complex stage – a larger team is required with the following functions:
- Process development
- Product development
- Analytical development
- Manufacturing
- Quality
- Regulatory
- Medical professional
- Facilitator
Step 2 – Identify quality attributes
Second, you need to dentify and understand which materials and parameters affect the Quality Attributes (QAs) is key. Acknowledging and understanding the qualitative and quantitative extent of the impact is also critical for maintaining manufacturing excellence. These Quality Attributes must be in the top row of the RAMM analysis.
Step 3 – Define the process steps
Third, you need to define process steps. At their core, process steps convert material into the final product. For example, a filling operation has many similar process steps no matter what the country or region. Therefore, they are a global “must” in the risk analysis process.
Take the filling process, for example. The block below is a standard process diagram. Though these steps will be fully integrated, this is a necessary step towards a rational analysis of the risks in this production.
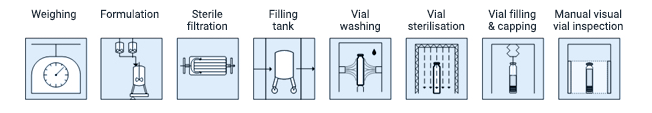
Figure 2. Simple description of process using process block diagram.
Step 4 – Define the process and material
Once the overall process is defined, you need to break down each process step into process parameters. Analyze each process block to determine which inputs or factors to that process step are present. These inputs are represented by an arrow pointing toward the box identifying the process step. Process responses or outputs are depicted with an arrow pointing away from the process step.

Figure 3. Process diagram demonstrating process inputs and process outputs.
Step 5 – Create and score the RAMM
The RAMM uses a matrix of process input factors and quality attributes to assess risk and impact. The axis of the quality attributes (top row) and the process (left columns) form the basis of the RAMM.
Assigning stratified scores – one for factors posing low impact or risk, three for factors posing moderate impact or risk and nine for factors perceived as having high impact or risk – also facilitates identification of the important factors and speed. The unimportant risks or parameters receive a low score. The items in between are assigned a moderate score.
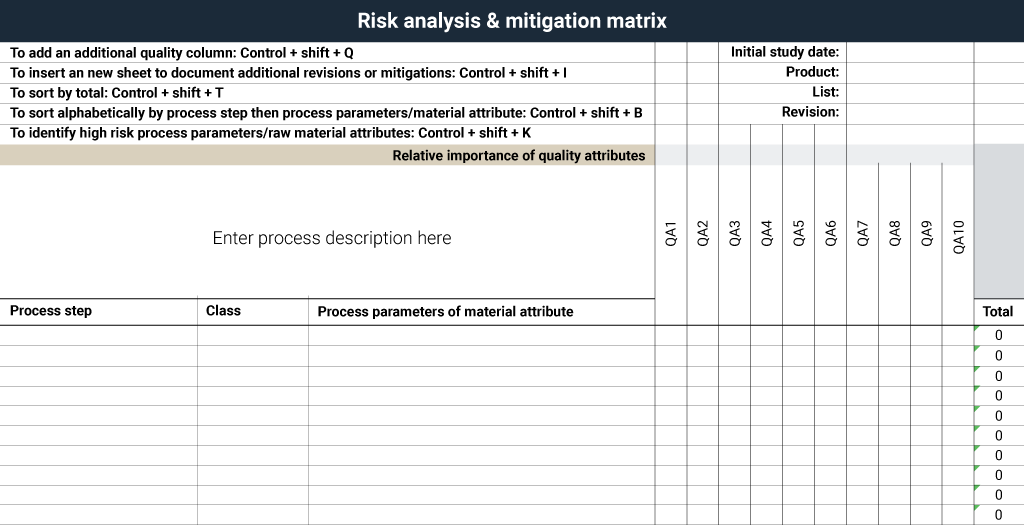
Figure 4. Empty RAMM template.
RAMM forces users to consider the most important items that might fall by the wayside in complex processes. By weighting the important items, they rise to the top and demand attention.
At this stage, you can sort the RAMM and filter the process inputs to assess them by their impact on: a specific response, the interim process step, or their overall impact on the entire process. The matrix can be filtered further to determine which process step or input factors have the greatest impact on a particular attribute.
The order of cross products for both the rows and columns are “gut-checked” with the cross-functional team to ensure the scoring matches with their perceptions of the process.
Step 6 – Mitigation
Finally, there is mitigation This complete, thorough and rapid process allows for ongoing prioritization and mitigation to continue. Main risk items can be addressed and the exercise repeated as risk is minimized.
The advantages and disadvantages of using RAMM
The advantages of RAMM during risk management centre on the provision of a comprehensive and itemized overview. For example, RAMM:
- Neatly handles QA and CPP parameters in a single document and is therefore easily aligned with latest guidance
- Is fast - by overlaying QAs against CPPs, it is relatively straightforward to set up and rank
- Is detailed
- Gives an excellent overview - an entire process can be printed on just 1-2 pages
- Is easy to incorporate into a quality system, follow up on and use for documented changes
However, there are some disadvantages with RAMM.
- It can be challenging to align the team, particularly if they lack knowledge of risk analysis. This is especially true of scoring numbers as the individual risks or a combination probability and impact
- If the process is not well understood, the team needs enough experience to realize that some pre-work identifying parameters (such as p-diagrams) is required
These can be resolved with training and staffing more experienced employees.
Overall, all risk tools have value in a risk management system, but RAMM is perhaps the ideal compromise. It offers the most favoured combination of speed, detail and overview of the continuous manufacturing process.


