The worldwide sustainability trend is thriving in the pharma industry and all large companies now have defined goals. So how can you ensure new and existing pharma facilities meet these targets?
Whether it's a greenfield facility or rebuilding a ventilation system, it can be difficult to maneuver through endless possibilities and end with a sustainable outcome. In the pharma industry, GMP is always the main driver and it is thus important to focus on sustainability from the beginning to find good, integrated solutions.
Building a truly sustainable business is not easy. But working with sustainability presents as many opportunities as it does challenges. Indeed, sustainability is not about making things more difficult – it should be seen as a way of thinking and behaving to ensure facilities are designed and built for the future.
So how can you implement sustainability solutions effectively in a project?
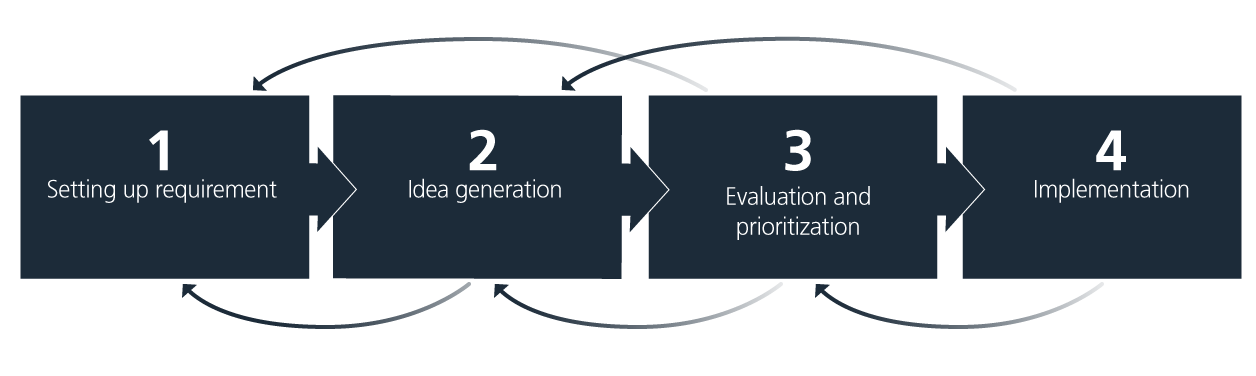
A structured approach that ensures continuous progress is key. The simple four-step model, illustrated and explained below, is a helpful tool to guide the process of integrating sustainability measures.
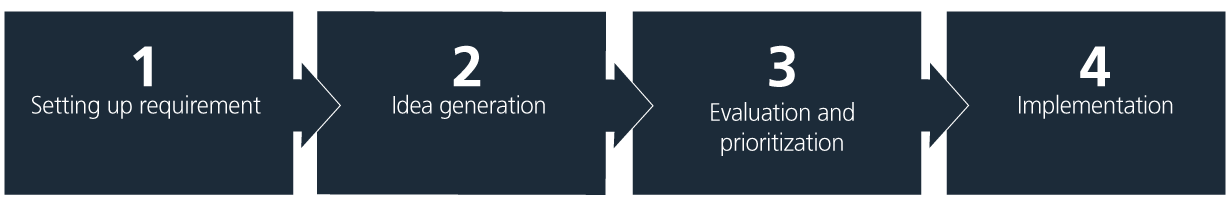
1. Setting up requirements
Before you start to develop and evaluate ideas, you need a good foundation. Everyone involved in the project must know its objectives and challenges. And depending on the ambitions and character of the project, these can vary significantly.
For example, is there water scarcity in the pharma facility's vicinity? Does your company have specific goals for reducing energy consumption? What is most important - investment or operational cost? For some, social sustainability, such as biosafety or the indoor climate, is more important than environmental impact. But ultimately, the targets you decide must be quantifiable so you can compare different ideas.
You should also consider if the facility is aiming for a sustainable certification system (e.g. LEED, BREEAM or DGNB) and which award you want to strive for (silver, gold, or platinum). Having a sustainability certification in mind can ease the process as the point system and requirements are already defined.
2. Idea generation
Depending on the scope, size and character of your pharma facility project, different approaches can help generate ideas. In smaller projects, idea generation can often be decentralized. However, in larger projects that involve multiple stakeholders and professional disciplines, a workshop-based approach is typically the most rewarding method.
In this phase, it is important to address and document all ideas, including ideas that are already implemented and ideas which – for some people – may be common sense. All the ideas will be evaluated later and used to illustrate what has been done to accommodate the targets of the project.
Two important things to remember in the phase:
- Sustainability ideas, including subjects such as energy optimization and water reduction, are very complex when it comes to GMP. It is thus important to activate all expertise areas and engineering disciplines with your chosen engineering partner.
- Optimization doesn't always mean new technology or procuring expensive equipment. The best and most rewarding initiatives are often free and only concern reduction for e.g. indoor climate or room classifications.
Once all the ideas in this early phase have been generated, they should be collected in a catalogue. This can be added to throughout the entire project when new ideas arise.
3. Evaluation and prioritization
With a catalogue overflowing with ideas, it is now time to do the first cleanup. This is done by categorizing each idea with a green, yellow or red label:
- Green: “Implemented” or “must be implemented”
- Yellow: “Investigation is needed” or “has not been decided on yet”
- Red: “Not implemented” or “declined”
Naturally, in the beginning of a project many ideas will be yellow since a lot is unknown. The goal during the project is to change all yellow ideas to either green or red.
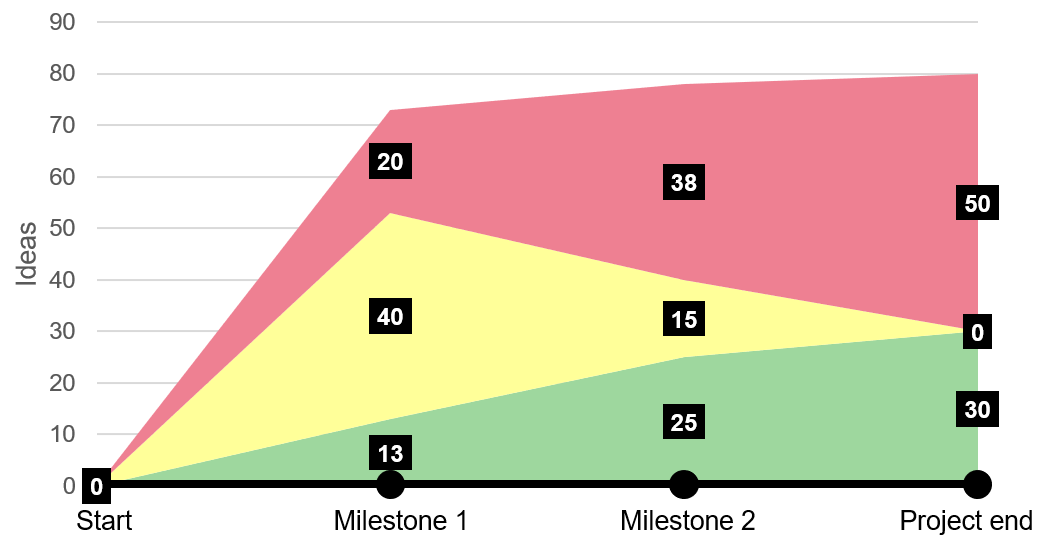
It is important to recognize, however, that not all ideas can change to green, since many ideas will have the same purpose or contradict one another. Here, evaluation is a helpful exercise.
When evaluating an idea, it is vital to have a basis for comparison. Are you comparing different solutions that solve the same challenge e.g. choosing between different chiller solutions and combinations? Or is the idea to upgrade an already implemented solution to be more energy-efficient e.g. adding an economizer to a steam boiler?
Either way, each idea needs to be evaluated against a baseline. With the economizer example, the baseline is the efficiency of the steam boiler without the economizer. The comparison can be made on the operational cost, CO2 emissions, operational reliability, payback period or other relevant parameters depending on the requirements defined in step 1.
A big challenge when working with greenfield projects is how to set up a baseline for documenting the environmental improvements in the project."
But with what can you compare a new facility? A big challenge when working with greenfield projects is how to set up a baseline for documenting the environmental improvements in the project. In a renovation project, you often have before-and-after data on energy and water consumption. In greenfield projects there is no "before".
The best solution is to define a baseline facility that contains your normal procedures, e.g. good engineering practice and regulatory requirements initiatives, but none of the extraordinary sustainable initiatives you wish to implement. This baseline calculation represents the “before”. Then a new calculation is made where all additional initiatives is included.
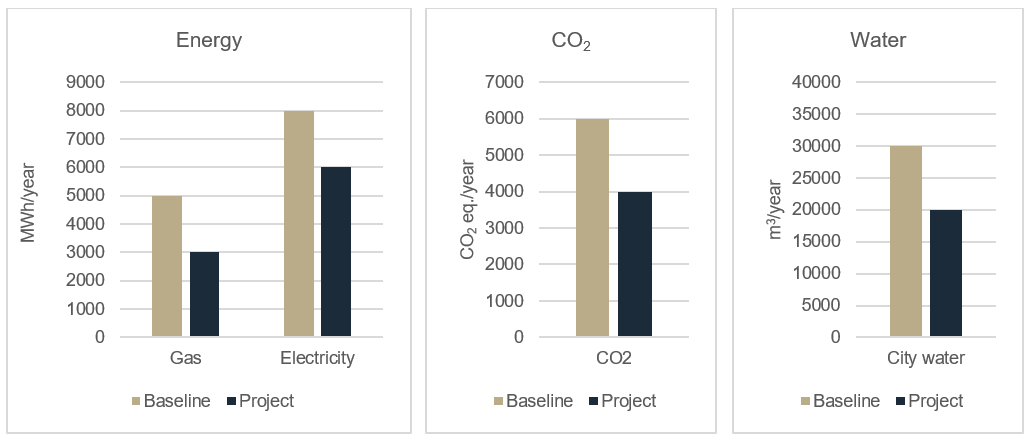
Fig. Energy consumption, CO2 equivalents and water consumption in a greenfield project compared to baseline.
4. Implementation
Good intentions and thorough investigations alone are not enough to ensure the final project includes the best sustainability initiatives. Ideas can easily be lost when changing to a new project phase, especially if new people are introduced or if the project team is working in silos.
When working with pharma projects, GMP is the core focus together with national authority regulations. The idea catalogue must thus be followed up on and updated regularly. This ensures that any "decided" ideas are revisited and new ideas are documented and investigated.
The linear approach described is not fully representative, but should be seen as an iterative process. New knowledge and changes during the project can change the prerequisites for initiatives and a revisit could be needed. Finally, although it gets more difficult to implement changes in a project as time goes on, new ideas should still be welcomed during the whole project phase.