'One-size fits all' no longer applies to the world of pharmaceuticals. A growing understanding of genetics, the immune system and the potential for stem cells means more effective treatments and cures for severe diseases.
Today, personalized medicine is already significantly advanced. Research now shows that with generic medicine, some patients benefit, others obtain no effect at all, and some even experience adverse effects. The ability to differentiate treatment between patients – even patients with the same disease – is drastically changing the pharmaceutical drug landscape. And in doing so, it has shifted the way medicines are manufactured.
One example of tailored therapies for individuals are “autologous therapies” where a unique patient is treated with their own unique in vitro modified T-cells. Autologous oncology therapies are a major game changer. For example, chimeric antigen receptor cell therapy (CAR-T) initiates a bioengineered immune system attack on cancer.
When the FDA approved Novartis Kymriah CTL019 in August 2017, it was a groundbreaking moment in the development of these drugs. It was the first gene therapy to treat pediatric and young adult patients with B-cell acute lymphoblastic leukemia (ALL).
Since then, the FDA has approved several clinical trials for advanced therapy medicinal products (ATMPs) and now 13 cell therapies are officially approved. Donovan Jones from IIR Life Sciences predicts that “The global market for cell-based therapies is expected to surpass $20 billion USD by 2025, with an annual growth rate of 21%.”
Although it is unlikely this new paradigm will completely replace larger manufacturing operations, this shift is here to stay. Currently, there lacks an all-in-one package for a sustainable and future-proof manufacturing concept, but manufacturing equipment is under development to become fit for commercial manufacturing purpose.
What does this mean for new or refurbished facilities in this segment? First, they need to be flexible, agile, and future-proof. Second, they should be extremely good at handling a small number of precious batches at a higher frequency to enable the manufacturing of unique, personalized drugs like CAR-T therapies.
Manual processing is prone to errors and is expensive in operations - in fact, it is said that up to 80% of errors in pharmaceutical production are down to human error.
Staying in compliance: GMP challenges in a personalized medicine world
If you are preparing to take a cell therapy into commercial manufacturing, there are some big questions to ask and decisions to make. Should you build your own manufacturing facility, use a contract manufacturing organization, or lease a cleanroom container until processes and equipment are developed further?
Whichever option you choose, the first step on this evolutionary journey is to consider the GMP implications and implement a strong control strategy.
Multiple manufacturing suites and manual handling
Producing autologous therapies require aseptic manufacturing suites. Unless the manufacturing area uses closed processes, this requires a high GMP classification. Additionally, most production suites use manual aseptic processing, which requires highly trained and qualified personnel. And as more therapies for more patients are produced, more manufacturing suites are needed to be able to produce batches in parallel. So it is a case of "scale-out" rather than "scale-up".
The main issue here is that manual processing is prone to errors and is expensive in operations - it is said that up to 80% of errors in pharmaceutical production are down to human error. This percentage hits particularly hard when you consider how these unique therapies use a patient’s own stem cells, so any unplanned shutdowns or faults can result in impurities in the cellular therapy product. This can be life-threatening for the patient, particularly in cellular therapy facilities where treatment is often acute.
In addition to this, Annex 1, one of the most comprehensive updates in EU GMP regulations to date, will be finalized this year. Among other things, Annex 1 focuses specifically on a general risk-based approach, moving the operator away from the product and making sure manufacturing processes are digitally traceable.
>> Read more on the Annex 1 draft from the EMA
A journey towards closed processes and full automation
So how do we deal with these challenges? Most importantly, we need a change in mindset. Although manual handling still dominates in the development of autologous therapies and the start-up of commercial manufacturing, the potential for product risk and the associated costs of high-grade GMP environments logically point towards closed systems like isolators. Isolators are easy to scale-out and mitigate cross-contamination risk, as “open handling” is performed in a closed primary barrier system.
Short term, this solves many of the above issues. But long term, we need to take this one step further. The FDA guidance on Aseptic processing says:
“Automation of other process steps, including the use of technologies such as robotics, can further reduce risk to the product” 1
To substantially lower the risk of operator contamination, the ideal environment would be a fully closed barrier system, no operator in contact with the product, and a robot that conducts all handling tasks. Alongside this, utilizing single-use technology and closed process philosophy would remove the hurdles of cleaning and decrease cross-contamination risk
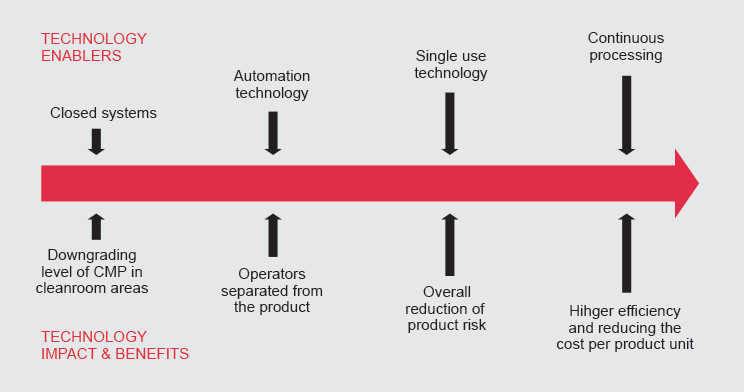
Sustainability benefits: operational cost-cutting and an improved working environment
The shift towards closed manufacturing processes also has other positive implications. For example, downgrading the GMP room classification by using closed systems significantly reduces the ventilation needed in a facility. Additionally, if the operator is separated from the product, it removes the need for a full gowning procedure several times a day. This results in leaner operations, reduced waste, and large energy savings.
Shifting towards this model also significantly improves the working environment for operators. Having to gown up and down several times a day, plus wear a full-cover suit and mask, is uncomfortable and makes it difficult to take regular breaks. A better working environment means happier employees, which means less stress and fewer errors. In addition, it makes your workplace more attractive to highly sought candidates within a very competitive industry.
Disruptive innovation in pharma?
Game-changing trends such as CAR-T cell therapy are sending waves across the manufacturing industry. ATMP facilities are moving away from manual handling and operators handling products in GMP grade A benches with grade B cleanroom backgrounds, to more flexible facilities with automated processes and operators separated from the product.
Just as disruptive innovation has affected numerous other industries over a short period of time - think LPs to tapes to CDs to smartphone apps - disruptive innovation also affects pharma manufacturing. Currently, the technology exists, but not in a convenient “all-in-one” package. Nevertheless, the speed at which innovative technology evolves has increased at an unprecedented pace, and pharma manufacturing technology is no different. The real question is not “if”, but when and how we will implement the changes.
Finally, to develop these concepts properly, we need coordinated cooperation between pharma engineering consultants, universities, hospitals, equipment developers, pharma manufacturers, CMOs, and regulatory bodies. If done collectively, what seemed unrealistic only 10 years ago could be a real possibility. With personalized medicine and autologous therapies taking the stage, sustainable ATMP facilities are now in sight.
1 Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice, Pg. 10


