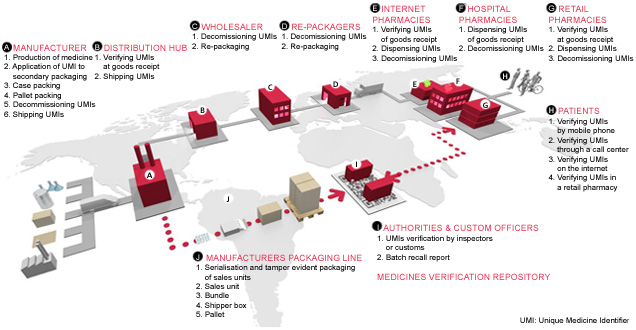
NNE offers consulting and engineering services covering all aspects of serialization in pharma manufacturing, from packaging to the point of dispense. We will help you build end-to-end serialization solutions for global compliance by providing consulting and engineering support within:
- Management consulting
- GMP compliance
- Automation and IT
- Serialization software
- Finished products
- Logistics and warehouse
With you throughout your serialization projects
We will take turnkey responsibility for your serialization project, and stay at your packaging site until the serialization solution works perfectly and you have reached your OEE target.
NNE has an insider understanding of pharma and biotech production and we are highly experienced in working with large complex systems such as SAP object event repository (OER), as well as other SAP modules supporting the supply chain. We can run projects from consulting through implementation to on-going support, leveraging proven methodologies and adhering to superior quality standards.
We work based on a proven model, which provides an effective method for structuring a serialization project with minimal business risk for the customer.
A 360º approach to serialization
NNE will help you define and execute serialization strategies that incorporate all your drug manufacturing processes and business processes. From IT through GMP compliance to the finished product, we can help you build an effective serialization strategy that not only improves your processes but creates cost efficiency.
Automation ans IT
|
Serialization software
|
Management consulting
|
Finished products
|
GMP compliance
|
Logistic and warehouse
|
Linking regulation and manufacturing
We work closely with government bodies responsible for creating anti-counterfeiting and compliance legislation, including the US Food and Drug Administration (FDA), The European Federation of Pharmaceutical Industries and Associations (EF PIA) and the Danish Association of the Pharmaceutical Industry (LIF).
Our first-hand experience with legislation and our experience in biopharmaceutical manufacturing enable us to pinpoint how the two impact one another. We can thus help you to interpret regulations and determine exactly what changes you need to make to your packaging line to achieve compliance. We enable you to comply with the rules as they come into force and to implement sufficiently flexible and scalable solutions.
IT technology and infrastructure will play an important role in meeting compliance challenges. Our IT expertise will make sure that you never invest in inadequate or incorrect IT solutions. And our first-hand regulatory expertise will ensure that you meet compliance challenges as they arise.
Driving cost-effective secondary packaging with serialization
A key concern for pharma manufacturing companies is that the large investment and increased production cost associated with implementing advanced serialization solutions will increase unit cost. This is a very valid concern. However, the global serialization legislative changes may be the best possible starting point for improving your packaging process.
At NNE, we have the competencies to make sure serialization implementation does not have a negative impact on unit cost. In fact, we believe that producing products more cost-effectively should be the main driver when you upgrade your serialization – and not compliance in itself.
Making your packaging more efficiently is an enterprise-wide commitment. From choosing the right serialization software vendor to designing or retrofitting your production packaging line, our technical engineering and integration services expertise make your pharma manufacturing processes more cost effective.
Our serialization services for secondary packaging include:
- Development of serialization strategy and implementation roadmap
- Line retrofit and systems integration analysis
- Project management for serialization
- Configuration and implementation of packaging line serialization software
- Automation to increase capacity
- Performance optimization via advanced control
- Vendor coordination and contract packaging mapping
- Product distribution and flow mapping