No matter the established success of a pharmaceutical product, regulatory authorities still expect continued process verification of these hot drugs. But if you haven’t established how to monitor the process before, how can you obtain the necessary process understanding?
Successful pharmaceuticals don’t happen by accident. Many of the market’s hit pharmaceuticals, or “legacy products,” have been developed long before the process validation guidance from FDA was published in 2011. However, health authorities expect companies to perform continued process verification (CPV – stage 3) on these legacy products regardless of their lengthy establishment.
By performing CPV through active statistical process control (SPC) instead of merely a monitoring effort in the production, you can achieve huge business benefits beside “just” compliance. Typically, processes have not been developed for CPV, which often means that companies have not specified how to monitor the process with critical process indicators (CPI) nor how to act on the monitoring signal with critical process parameters (CPP).
However, filling this knowledge gap can be as simple as 1-2-3. Attain the necessary process understanding and subsequent ability to the control the process in three steps.
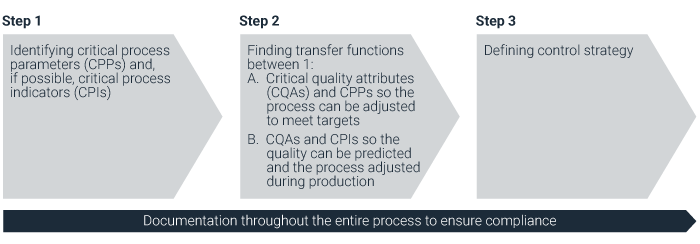
Figure 1. The 3-step process continuous process verification.
Step 1: Identify critical process parameters
If you do not know your potential critical process parameters (CPPs) you simply cannot establish a robust controls strategy. In practice, this determination can be performed in a half-day workshop with participants representing all relevant stakeholders such as those in development, production, support, etc. It is essential to select a broad group of participants to ensure that they represent all relevant historical knowledge.
The workshop serves as a brainstorming session where the group prioritizes the critical quality attributes (CQAs) followed by a brainstorming on potential CPPs, which are then scored on:
- Importance for CQA(s)
- Lack of control
- Lack of detectability
- Ease of change or data collection
With this method, you can potentially convert 100 possible CPPs to a prioritized list of less than 10 CPPs.
Step 2: Find transfer functions
Transfer functions are solid mathematical correlations, such as those between CQAs and CPPs. Once you have prioritized your CPPs, the next step centres on collecting data on said CPPs and their corresponding CQAs to find the transfer function between them.
If historical data is insufficient in quantity and/or quality, supplementary data must be generated. This can be done with a design of experiments (DoE) approach through either:
1. Evolutionary operations (EVOP): varying few CPPs in small steps within existing process limits, which still allows batches to be sold, or
2. Full DoE: involving more factors and larger steps where batches will (often) have to be scrapped
Both alternatives offer the unique possibility to identify CPIs that can predict CQAs, done simply by measuring both CQAs and CPIs on all performed experiments. In a full DoE-approach, CPPs are heavily varied, resulting in large variation in both CQAs and CPIs, making it easy to identify the correlation – if there is one. Whereas with historical data, there is typically not enough variation to identify any clear correlation. If there is a clear correlation, requirements for CQAs can be converted to requirements for CPIs.
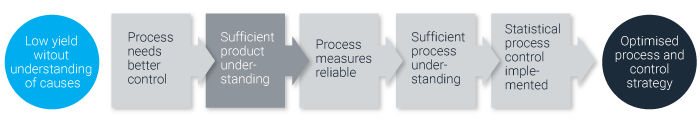
Figure 2. The journey towards an optimized process and control strategy.
Step 3: Define control strategy
In the third and final phase, the knowledge gained in steps 1 and 2 is utilized to devise the control strategy. The control strategy defines what to monitor, which by default are the CQAs, but if either evolutionary operations (EVOP) or a design of experiences (DoE) has been performed, this can be performed based on the CPIs. Additionally, the control strategy defines how to react on CPIs, based on the transfer functions.
If the data is sufficient, the long-term goal is in-line release, wherein you need not wait for confirmation of final CQAs.
By following these three steps, manufacturers will be able to get a better understanding of their legacy products and processes, thus enabling them to monitor the critical process indicators (CPI) and thereby ensure a timely reaction by acting and adjusting the appertaining critical process parameters. Understanding these relationships is essential when developing a control strategy, and will in most cases result in robust and improved processes and increased yield.