With the ever increasing potency of active pharmaceutical ingredients, implementing containment and closure within pharmaceutical manufacturing is a matter of how, not when.
In the pharmaceutical industry, traditional oral solid dosage (OSD) medicines account for approximately two-thirds of all pharmaceutical products worldwide. However, this value speaks more to the sheer volume of products than the financial turnover. So-called blockbuster drugs with high market potential and mono-systems are decreasing as blockbuster patents run out.
Active pharmaceutical ingredients (APIs) have become stronger and increasingly more active in recent years. This increase in potency is catalysing an industry transformation. These drugs need high containment facilities to avoid cross-contamination - important to protect the product, operators and the environment, particularly in the manufacturing of high potency active pharmaceutical ingredients (HAPIs).
Additionally, continuous manufacturing equipment is excellently suited for such products. Continuous systems do not require manual intermediate steps, such as transferring an intermediate product into an intermediate bulk container (IBC) from one process step to the next, thus minimizing the risk of cross-contamination. Even global regulatory authorities such as the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) have become increasingly restrictive and stringent, requiring additional methods, machines and building requirements to meet these requirements.
What is containment and how much is needed?
In the case of manufacturing, the term "containment" refers to limiting the spread of a substance or an agent and the respective shielding of the environment from loads by active substances and adjuvants. Containment comprises all measures that limit the spread of highly active substances including very simple substances.
Containment is always relative, meaning it does not refer exclusively to "closed systems." In fact, totally closed systems do not exist. Therefore, the question should not be, "Is containment necessary?" but rather, ‘How much containment is required?"
Containment covers a wide field of application and, as of yet, no uniform rules are available. It wasn’t until November 2015 that the International Society for Pharmaceutical Engineering (ISPE) in Germany, Austria and Switzerland published the Containment Manual in a containment seminar. Just four weeks after the presentation of the manual, suppliers and users in the industry utilized it as the new standard and reference.
Closed processes are not necessarily identical with a complete containment isolator. Depending on the potency of a substance, it might be sufficient to integrate a light or simple containment into the production process.
As a rule, the occupational exposure limit (OEL), occupational exposure band (OEB) and ISPE’s Category (CAT) system are used for the production of potent substances. They are divided into five different categories (see figure 1). The different use of these categories by equipment manufacturers’ and pharmaceutical companies’ can lead to possible uncertainties or incorrect assumptions, especially if these are not compared and defined at the beginning of a project.
In addition to the class, volume or category of an active substance, the OEL, permitted daily exposure (PDE) or acceptable daily exposure (ADE) value must also be included in the configuration. You should be able to achieve a harmonization of these categories by using the containment manual.
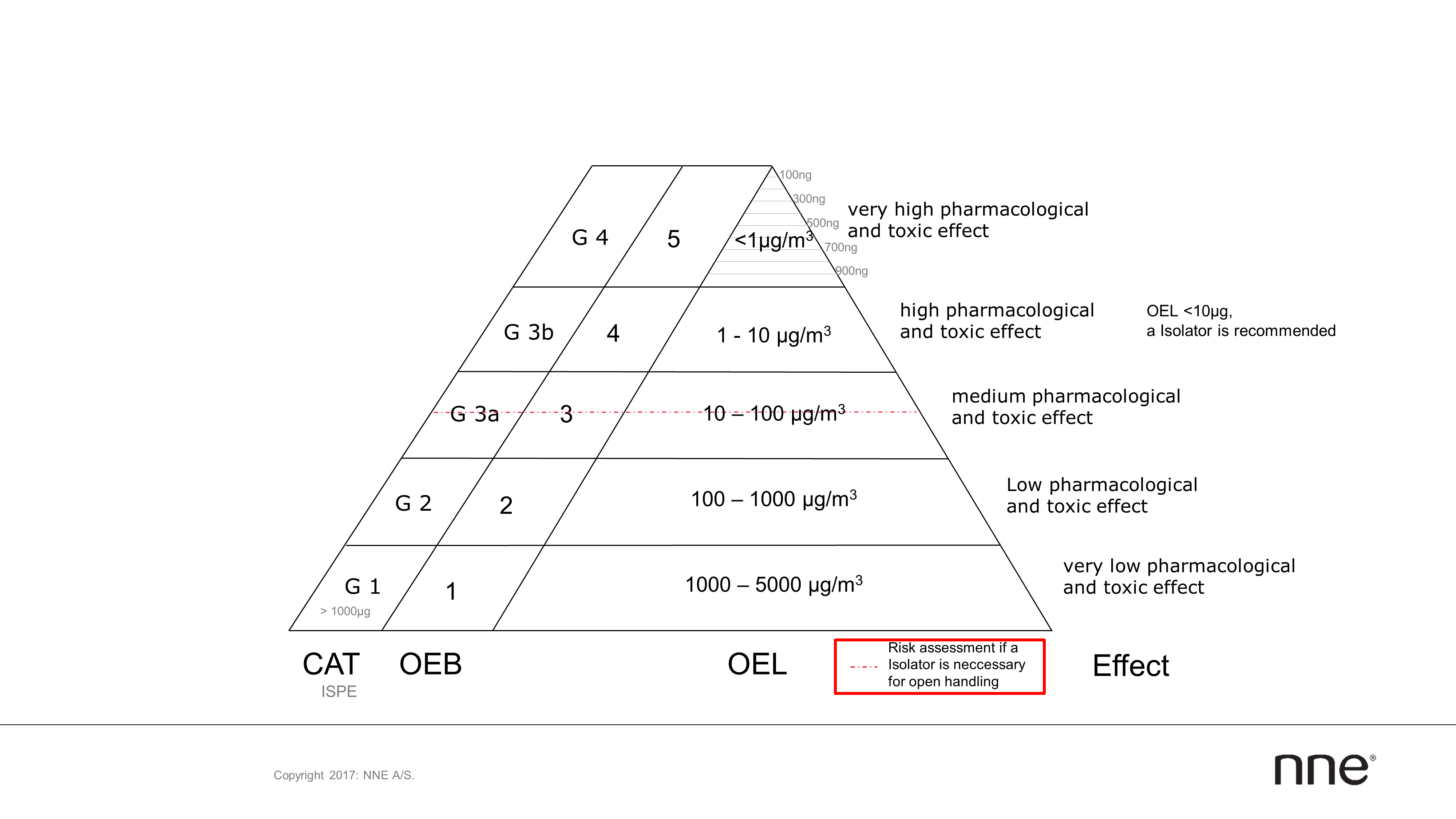
Figure 1: A classification system and containment triangle (OEL / OEB / CAT) of different systems.
The observations of the containment described here relate to solids in powder form, where possible absorption into the body takes place through inhalation. In order to conceptualize the amount of active substance in a room, imagine an Olympic-sized swimming pool measuring 50x25x2m with a volume of 2500 cubic metres. If a pinch of salt of 2.5 mg or 25 salt grains is tossed into this pool, a concentration of one microgram per cubic meter (1 μg/m³) is obtained. This corresponds to the highest containment level.

Figure 2: Quantity product with an OEL value of 1 μg/m³ in an Olympic swimming pool (2,500 m³) in 8 hours.
This reaffirms the point that the question is not "is containment necessary?", but rather "how much containment is required for the production of a potent drug?". The growing awareness of workplace limits suggests an increasing trend towards closed production processes. In fact, in the not-too-distant future, no open processes will be allowed in order to protect the operator and the product – even for non-potent drugs.
Levels of containment
However, closed processes are not necessarily identical with a complete containment isolator. Depending on the potency of a substance, it might be sufficient to integrate a light or simple containment into the production process.
Examples of light containment include a double flap or a closed container where a closed line, hose or pipe transfers raw materials. In addition, a tablet press, which is closed and fed closed, can be a simple containment. Tablet processes can be integrated into product transfer lines with filters that can be cleaned. Shields on process unit or process machines can be integrated with washing or cleaning in place (WIP/CIP) to prevent contamination of the operator when opening a dusty machine. These simple containments may be sufficient to ensure a higher degree of protection.
A new trend runs parallel with closed processes - the continuous production of tablets. This process boasts advantages, including production efficiency and a reduced good manufacturing practice (GMP) footprint as it requires a smaller area for setting up a process in a factory. In this manufacturing process, all processes are carried out, including automatic weighing, granulation, drying, mixing of binders, tabletting and coating in a closed system in the same room or process plant. Compared with batch production, this can reduce the required production space by up to 70%.
In addition, continuous tablet production enables the use of continuous production machines for the production of potent substances, as there are no manual transfer and transport steps of the product into a container between the process steps. Since all process steps are independent of one another, this also minimizes risk to the operator or possible cross-contamination with other products.
Figure 3 demonstrates a comparison of the process sequences for a classical batch production compared with continuous production. The only concern could be a possible breakdown, where a machine must be “opened” to break the containment and to clean or dismantle and exchange some parts quickly. The break of the containment in case of a breakdown should be considered in a risk assessment and is described as “the ring of concern”.
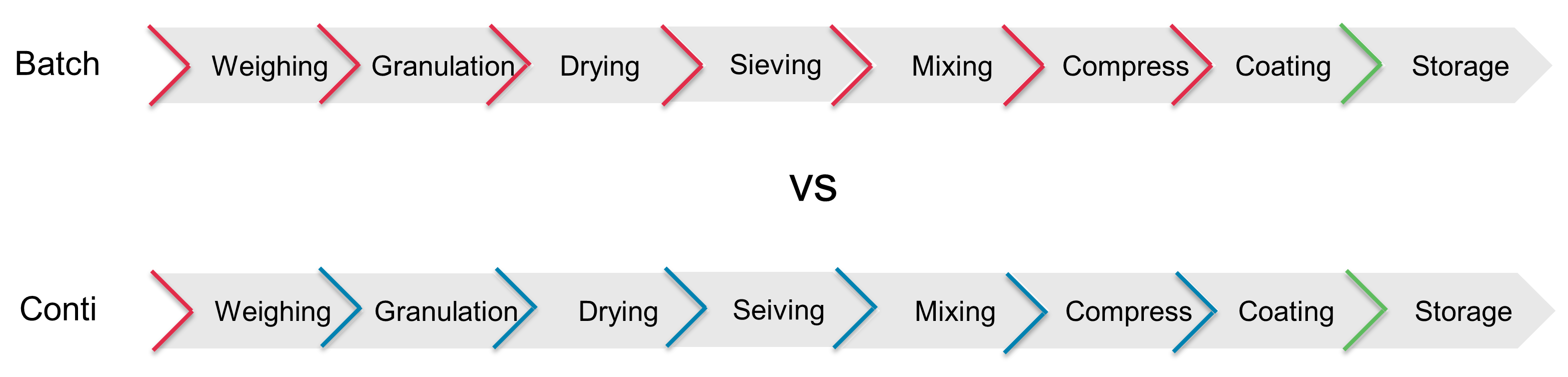
Figure 3: Comparison of a classical batch production with a continuous process. Process steps, in which contact with potent substances is possible, are marked in red. Intermediate steps, which are in-line and closed, are marked in blue. The safe end product is marked in green.
When it comes to handling highly active substances in the pharmaceutical industry, it is necessary to evaluate the extent of the containment correctly and to understand the consequences of insufficient containment measures. All substance potency factors such as the building requirements, ventilation and, above all, process and process management must be included in the safety considerations.
In addition, manufacturers must look to the present and the future. The containment concept should ideally consider having a higher safety rating than is required for the corresponding product to allow for future adjustments. In the future, open production processes will no longer be possible or even approved for the production of substances with increasing potency. It is clear that continuous production technology integrated with closed processes offers advantages over conventional batch production, particularly when producing smaller volumes which endanger the operator and reduce production space.



