After years of hesitation, the pharma industry is slowly beginning to embrace continuous process (CP) manufacturing. Indeed, after realizing the technology’s potential to cut costs, save time, and improve quality, pharma companies are much less reluctant to adopt CP than just a few years ago. But people and the way they work are a vital part of creating lasting change. So how can companies encourage operators to move from a "me" culture to a "we" culture in pharma facilties?
To fully harvest the benefits of CP, it is necessary to think about a pharma facility and its operations in an entirely different way. Furthermore, it requires a new skill set from the people involved.
One approach to a more widespread introduction of CP might be to forget about the technical challenges for a moment and focus on the people aspect. This calls for a typical change management approach with emphasis on the following two key exercises:
- Definition of current state and future state
- Stakeholder management
These two exercises are interlinked, as the work of defining the current and future state supports and facilitates that all stakeholders understand the purpose of activities, have a shared vision, and feel confident about the transition and endpoint of another way of working in a continuous setup.
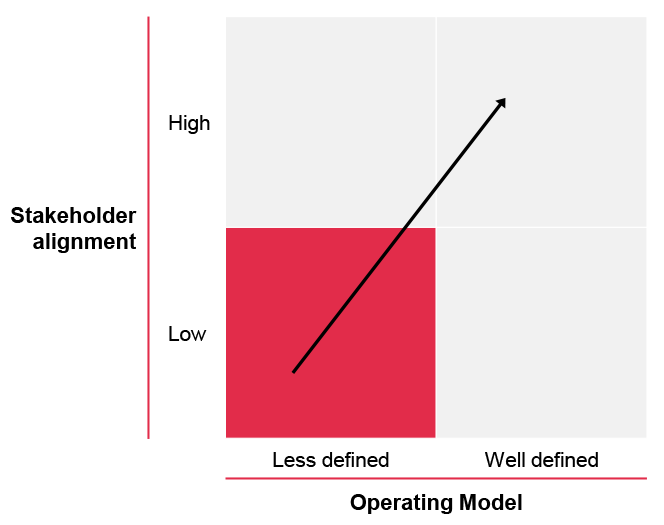
Illustration 1. Focus on defining the operation model and ensuring stakeholder alignment can enable the move to an improved performance stage – the future stage.
Moving from a “me” to “we” culture with continuous processing
One way to look at the current state and the future state is to view it as a transition from a “me” culture to a “we” culture. Illustration 2 below lists some of the elements and differences between the two cultures.
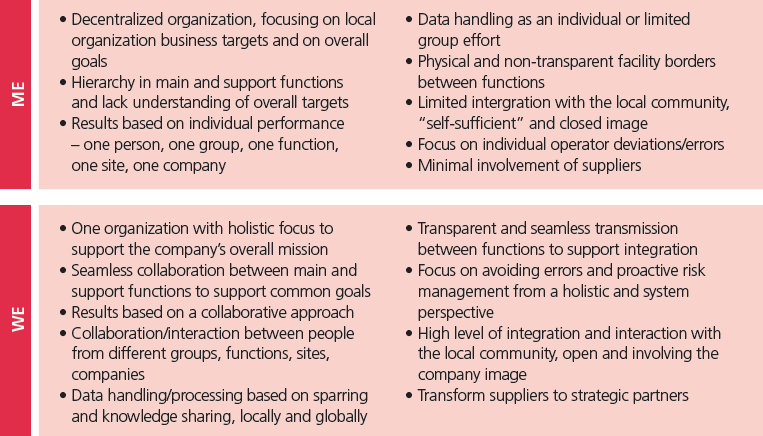
Illustration 2: Changing the way culture is viewed.
Pharma faces challenges today such as fierce industry competition, planning for unknown needs, and changing regulatory requirements to remain competitive. Attracting and retaining talent is an important part of being and staying competitive in today’s pharma business environment.
One of the traits of an attractive workplace is the ability to embrace diversity in work functions and to make operators feel like an important part of a bigger picture. The culture of a future company should include transparency in how contributions from individual operators create value for the bigger picture. At the same time, new ways of working should support a highly attractive, efficient, and integrated workplace.
But how can you facilitate a culture that fosters local/global collaboration and the fast, efficient sharing and transfer of knowledge?
First, companies should establish new types of workspaces such as interactive collaborative hubs as an integrated part of new state-of-the-art facilities. Smartboards, touch screens, data handling, and global platforms are key elements in such hubs.
Another focus area to create an attractive workplace is to develop an environment that supports simple and seamless movement between functions, and at the same time, creates transparency, cohesiveness, and supports people interaction.
Finally, it is vital to attract people with thorough process experience and who are confident in working with systems and handling and extracting knowledge from huge amounts of data, to work with young talent. Such interaction supports innovation and continuous improvement because it allows companies to make better use of all the data generated during product development and manufacturing.
Applying visualization
A sound and detailed understanding of workflow and all process operations supports this transition and helps fully align all stakeholders. Tools like operational workflow diagrams (see Illustration 3) are instrumental in providing a shared picture of the current operational setup and function as a visual tool for discussing and designing the future setup. Additionally, it allows teams to challenge critical processes and make base assessments and recommendations on factual data, capabilities, and capacities.
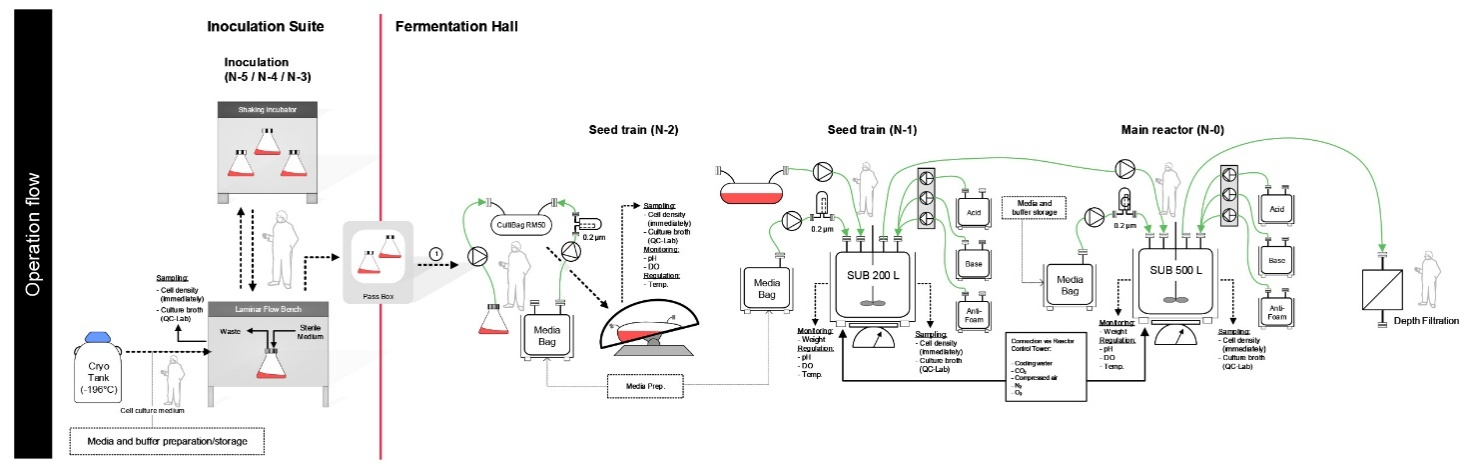
Illustration 3. Example of an operational workflow diagram for an upstream process
Generating flow
A key element of CP is to ensure continuous flow in the drug manufacturing process. However, the term “flow” might be an unfamiliar concept to operators coming from a traditional setup, where typically each operator is responsible for one operation or a set of unit operations. This naturally causes the operator to focus primarily on optimizing that particular unit of the operation or process.
The ideal CP manufacturing setup links the entire production process, from cell expansion to packaging, into one continuous flow. Moving forward, the key to success is not to optimize each operational unit – rather the attention needs to be on facilitating a seamless flow of the product intermediates throughout the process and the entire facility.
Operators thus need a holistic view and approach to continually monitor the entire process and search for potential bottlenecks or other factors that interfere, or might interfere, with a smooth and continuous flow.
Consequently, operators need to change focus from primarily performing manual operations to using the majority of their time on monitoring processes within operations. This includes:
- Surveillance of the entire core process and all supporting sub-processes
- Ensuring undisturbed and just-in-time flow of materials to be used in the process
- Facilitating fast and undisturbed flow data, process samples, and production documentation
- Interpretation of real-time process monitoring parameters and quality indicators
- Surveillance of automated feed forward and feed backward loops
- Adjusting the process when needed adjustments exceed the capability of the automatic systems
- Developing a sixth sense or intuition to predict and avoid situations that might interfere negatively on the flow before it happens, or in determining the cause of an interruption in the flow
Such competences can only be developed and work efficiently when the operators work as true teams, covering different competences and constituting a deep understanding of the entire process. In a biomanufacturing setup, the whole process includes all steps from the cell bank to the final drug substance or even drug product, as well as the sub-processes supplying the materials to the core process and handling the outputs. Outputs can include product, samples, waste, and data in many shapes and forms.
Designing the facility
Traditionally, the manufacturing process has been the starting point when commencing the design of a new facility. Moving forward, facilities need to be designed with a broader focus to include aspects related to operations once it has been established. The operating model includes decisions about areas such as automation level, logistics, flexibility, and most importantly, the control strategy.
With CP, reversing the sequence means a robust design, i.e. design begins with a definition of the operating model (or infrastructure) and from there and in parallel the design is developed based on different process scenarios. This allows the facility to accommodate the manufacturing of different modalities supported by the chosen operating model. The goal is to ensure early identification of requirements, well-defined interfaces, and a clear scope for the facility and the possible process.
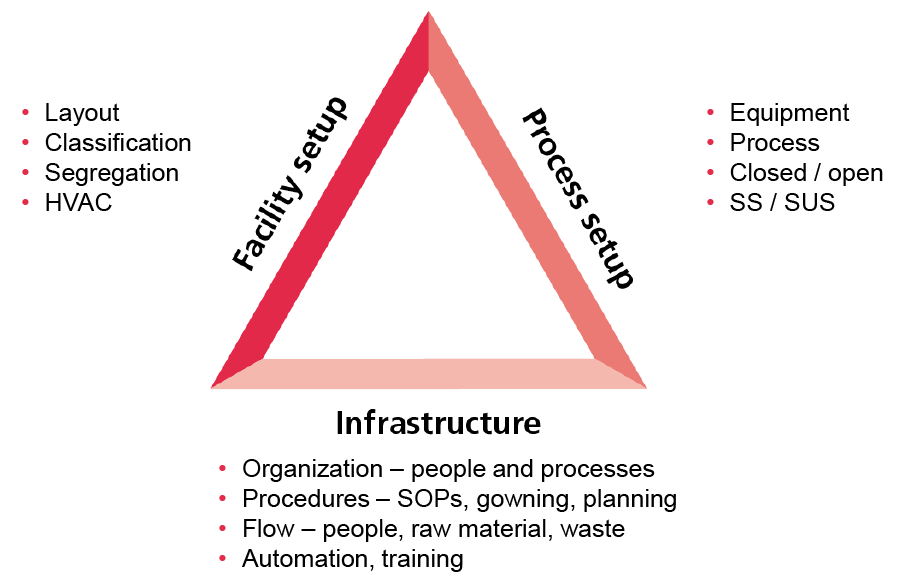
Illustration 4. The triangle of pharmaceutical facility design.
The model in Illustration 4 provides an approach to continuous pharma facility design that incorporates daily operations.
For example, a continuous operational model where equipment runs as a 24/7 operation for several weeks or even months is significantly different from a batch-based manufacturing setup with inevitable pauses between the batches. This entails that unplanned changes are a large challenge in a continuous facility, which is why it is critical to use a front-loading planning and programming approach to ensure a solid basis for decision when designing a continuous facility. This applies to establishing the facility as well as designing the operation model.
Another valuable tool to ensure flexibility in continuous manufacturing is to divide the process into logical operating units (LOUs). These LOUs should be offered to fit different process requirements in manufacturing suites and take advantage of convenient adjacencies where possible.
Flexibility allows LOU adjacencies to be defined based on the relative size of material flows and process requirements. This manufacturing approach requires that it is easy to move the LOUs in and out of the rooms, and that sufficient storage capacity is established adjacent to the manufacturing suites.
A “ballroom” concept would further facilitate such a setup, as it makes it easier to fit the different process equipment setups. However, it is then a requirment to ensure all the processing is performed in a closed setup. If any open operations have to be performed, they have to be done in isolators or in separate rooms.
Moving quality closer to manufacturing
Continuous processing requires that analytical and quality assurance support is moved into or at least close to manufacturing suites. This is a design driver that affects both the facility layout as well as the operation model, including moving to the we setup instead of the me setup.
In batch manufacturing, the product is produced and samples are sent for analysis, and when the quality requirements are proven to have been met, which might be hours, days, or even weeks later, the product is then released for further processing or for distribution to the market. Continuous processing, on the other hand, is based on real-time release. This means that all or most elements of quality assurance must be maintained throughout the entire manufacturing process. Accordingly, the design has to support analysis in real-time and enable quick responses to any deviations in each of the process steps.
Process analytical technology (PAT) and other types of advanced process models for multivariate process control, based on a high level of process and product understanding, are thus pivotal for successful continuous operation.
Maximizing automation and robotics
Continuous manufacturing is supported by automated processes to a much larger extent than batch manufacturing. Robotic solutions enable continuous manufacturing and can be applied, for example, to the following:
- Take samples from the process and transport them to quality control (where process analytical technology instruments take over and perform the analysis online, providing feedback to the control systems)
- Lift, empty, and generally handle large bags and containers
- Load and unload materials to machines. Finished pharma facilities have several load and unload requirements and often transport materials to and from production equipment. By allowing a robot to unload the filling machine and automatically transport it to inspection or freeze-drying, the production could be made continuous from filling to inspection
With robotic solutions undertaking these tasks, the role of human operators changes. Instead of operating the process, operators must be able to monitor the process. This requires a much deeper knowledge of the process setup. There can be challenges related to the technical complexity of the systems, which requires more knowledge of the processes and how to control the manufacturing processes. This increases the demand for proper training.
Building a robust control strategy
A final requirement to support successful implementation of continuous manufacturing is the development and definition of a thorough control strategy (see Illustration 7).
When a new molecule makes it through to CMC development, the prioritization and orchestration of development activities are essential to make it first to market and overcome regulatory CMC hurdles. A holistic control strategy supports speedy development, as it drives a systematic development process based on quality by design and makes it easier to focus and prioritize development activities within a cross-functional team.
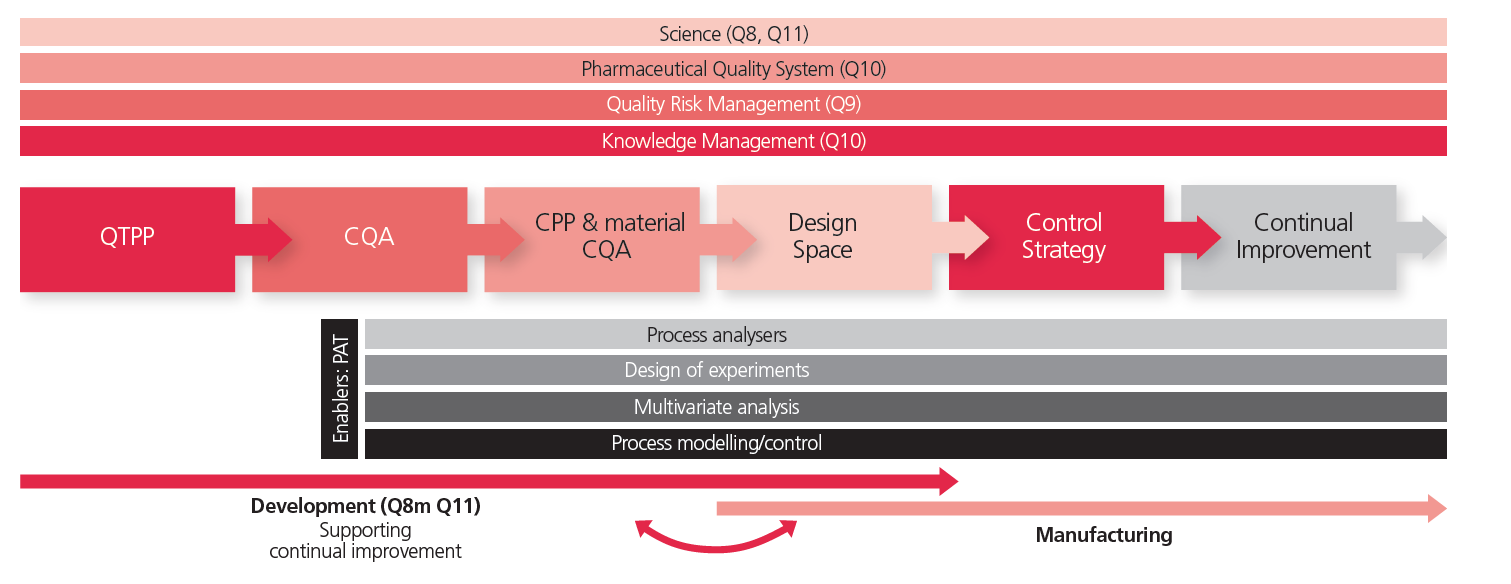
Illustration 7: The QbD principles as laid out by ICH Q8 and Q11 as an enabler for a speedy and systematic development approach those results in a control strategy.
In the development lab, a model or a number of model product and processes can be used to establish a generic control strategy, and each new product project must target specific development activities to where the control strategy might deviate from the generic model.
Development of a control strategy requires a structured process, linking biopharmaceutical development to the manufacturing process and engineering controls of process equipment. These activities must be supported by an innovative analytical and integrated data platform, and systematic development and knowledge management tools, such as electronic lab book, QRM, DoE, statistics, and multivariate software. These tools must be able to interact, for example,
so output from one tool can be used as input for another tool. Business aspects (control of yields, process time, energy consumption, downtime, etc.) can also be included in the manufacturing control strategy, making it even more powerful, as it not only improves product quality, but also ensures an optimal business performance.
Conclusion
Successful implementation of continuous manufacturing requires the industry to take a new approach when designing facilities. The mission must be to create a design that focuses on new design drivers and takes the operation model and the people aspect much more into consideration than when designing and operating a traditional batch-based facility.
This is an edited version of the originally written piece for the 15th Bioplan Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production. The full report can be purchased and dowloaded from Bioplan Associates's website.
Photo credit: The Ecoprime twin, courtesy of LEWA.


