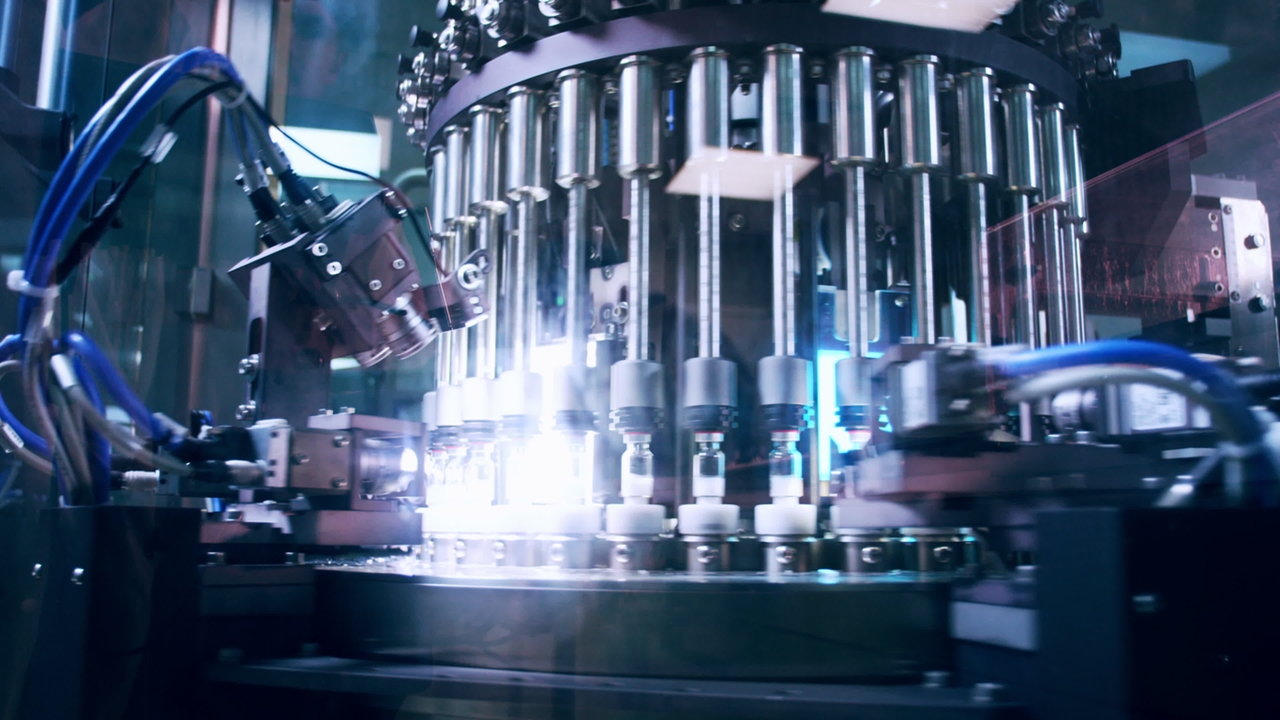
Fill-finish manufacturing is vital to create parenteral products that are safe and free of microbes. Traditionally, there were many manual steps in this pharma manufacturing process. But with personalized medicine, highly potent drugs and small batches becoming the new norm, we are looking at a digital, sustainable future with far more automated isolators and closed processes. And with constant new advancements, this journey is by no means over.
Current trends – what does a modern fill-finish facility look like today?
Current trends in the fill-finish facility landscape still surround protecting the operator, so single use systems are commonplace. Vials or syringes, for example, now come in container “tubs” that are fixed and separated from each other in nests and arrive closed, sterile, and ready to use. The nests make the vials or syringes less fragile and easier to handle automatically in an isolator, while also speeding up change-over between batches.
Robotic technology replacing gloves in isolator lines
Significant improvements in robotic technology have also led to fewer operators (and thus less chance of contamination or exposure). A cutting-edge pharma facility of today has isolator filling lines where vials or syringes are handled by robots and few interventions from bulky gloves that are difficult for delicate operations.
Even if a vial or syringe is broken, robots controlled by Artificial Intelligence (AI) and guided by a 3D vision camera can clean-up the mess. This positive step forward reduces a major source of contamination or operator exposure from tiny pinholes in gloves.
In fact, due to increased automation, fewer gloves are necessary in isolators. For the small number that remain, it is possible to dock testers onto the gloves themselves and test them in situ, rather than manually removing and testing them individually. The results can then be sent to a central computer that collects data and creates a batch report.
Inspection machines improved by automation and AI
Modern inspection machines are also becoming highly automated. In relation to the nests discussed above, some have integrated de-nesting, vision inspection and renesting as one integrated process. These machines can also reinspect false rejects of objects without interference from the operator.
Finally, AI is becoming more commonplace to continuously optimize the inspection process. Deep Learning of the system can teach inspection machines to decide whether it is particles detected in the product or a simple air bubble. This technological step forward reduces false rejects and increases the efficiency of the process.
The next generation of fill-finish pharma facilities
Cell and Gene therapy is a fast-growing segment of the pharmaceutical market, alongside a marked increase in vaccines to maintain diseases. Today, 50% of all chemical entities are classified as potent drugs and highly potent drugs will become more common, which will increase the need for equipment designed for high containment levels. Personalized and highly potent drugs also call for very small batches, which again increases the focus on reducing time for batch changeover.
So how does this affect the evolution of equipment in a pharma fill finish facility? Overall, we are looking at a digital, sustainable focused future with far more isolators and closed processes that are fully automated. Manufacturing will need to be flexible and easy to operate, with many small batches produced simultaneously under the same roof.
Digital twins, Artificial Intelligence and Machine Learning moves further
In this ever-changing landscape, an innovative mindset is crucial. Gathering Big Data will make it possible to examine the patient’s reaction to a particular drug and give analytical results to optimize the formulation. In addition, AI and ML will be incorporated into more and more equipment.
Digital twins of real-life processes on production lines will also make it possible to compare the two and enable equipment to quickly counteract small deviations. This will reduce the number of incidents where production is stopped because of an alarm.
Instead, these systems will adjust relevant parameters to keep the product within a narrow window of settings. Ongoing data collection will further refine these adjustments and “adapt on the fly” to reduce the number of rejects – extremely important with small, expensive batches.
Alongside this, pharma manufacturing equipment of the future will include:
- Single-use systems that work with continuous processes in formulation to help reduce or remove batch changeover time. Small batches will then run in the facility as continuous processes. This also means fewer operators, with no more than one in each formulation room.
- Smaller, automated filling machines. Instead of 1.200 syringes per minute, these will be very flexible and adjust to the product at hand. Or we will see multiple small, dedicated machines for each type of product standing side by side in one room.
- There will be more isolators with integrated wash down capabilities for self-cleaning. This will reduce the risk of a breach in containment facilities.
- More isolators will have point-of-use air filters in the return duct. If you can filter out potent drug particles before they reach the air-duct system, you save time cleaning in batch changeover – a precious resource when creating multiple batches.
- More isolators will have integrated AHU units. These take air from the room and filter it back inside the facility (rather than outside). This reduces the size of isolators and simplifies installation into existing buildings.
- New lyophilization technology will reduce the space need and energy consumption for freeze drying processes considerably. Currently, a 40 sqm freeze dryer might take over 60 hours to process a batch of dried product. Spray freeze drying can process the same amount of product in less than 24 hours. It also has space savings of 80% and a similar saving in cost. Furthermore, this technology makes it much easier to produce freeze dried product in syringes and cartridges, rather than conventional technology that mostly uses vials.
Finally, in relation to sustainable solutions, these will become more and more important. A holistic view will be key, with energy, air, water, raw materials, chemicals, and social impacts all being part of the equation.
Think wild – what could be the future of pharma?
What if we had a facility with an AI neural network, IoT for all process controls with real-time release of product, no human interventions and 24/7 operations? Or what if we could change the drug manufacturing process completely, where people can print their medication at pharmacies or even at home?
It might sound far-fetched, but similar technology is already being developed and used today. In 2020, for example, pharma manufacturer Takeda developed and implemented an automatic line-clearance system with 360-degree cameras, laser sensors and a dry run mode utilizing artificial intelligence.
Johnson & Johnson, one of the world’s biggest pharma companies, has worked for some years on 3D print technology of tablets, and recently FabRX, a British startup company, has develop a commercial sized 3D printer for personalized pills. The single step printing process manufactures pills with certain properties, such as sustained or delayed dose, and multidrug combination pills (polypills) with a multi-layer printed tablet containing several different drugs. This could revolutionize small batch production for clinical trials or the production of precise personalized medication for individuals.
Imagine – a patient with diabetes has a personal printer at home, containing all the ingredients for an insulin tablet. But the complexity of the disease requires different strengths depending on exercise level, diet and many other conditions. This patient could simply let the printer scan the iris for a few seconds, then decide on the perfect dose and print the tablet. With this, the patient will always have the perfect dose every time.
There are also developments for the administration of drugs – needleless injections, for example, already exist. “Smart pills” are also a possibility, where a small capsule with a reservoir of medicine is swallowed by the patient. The Smart-pill makes analysis of the body and releases the exact amount of medicine needed. This may seem sci-fi – but PillCam already develops ingestible capsules equipped with camera systems to visualize the digestive tract.
Clearly, we’re looking towards a high-tech future. And although it is not entirely obvious how it will pan out, it is an exciting time for the traditionally conservative pharma industry. We are witnessing the wind of change, with many innovations being implemented right now to optimize manufacturing and improve life for patients.