There’s no denying it - cell and gene therapies are about to revolutionise pharma. There are now triple the number of cell-based therapy trials compared to anti-body product trials. If just 20% of these become commercial products, this will have a huge impact on the industry, with companies needing to increase production capacity at speed.
Market competition means getting cell and gene therapies to market first and fast
The financial perspectives are very promising for cell and gene therapies (CGT). Development within this segment is growing rapidly, and the trends seen in the graphic below continue.
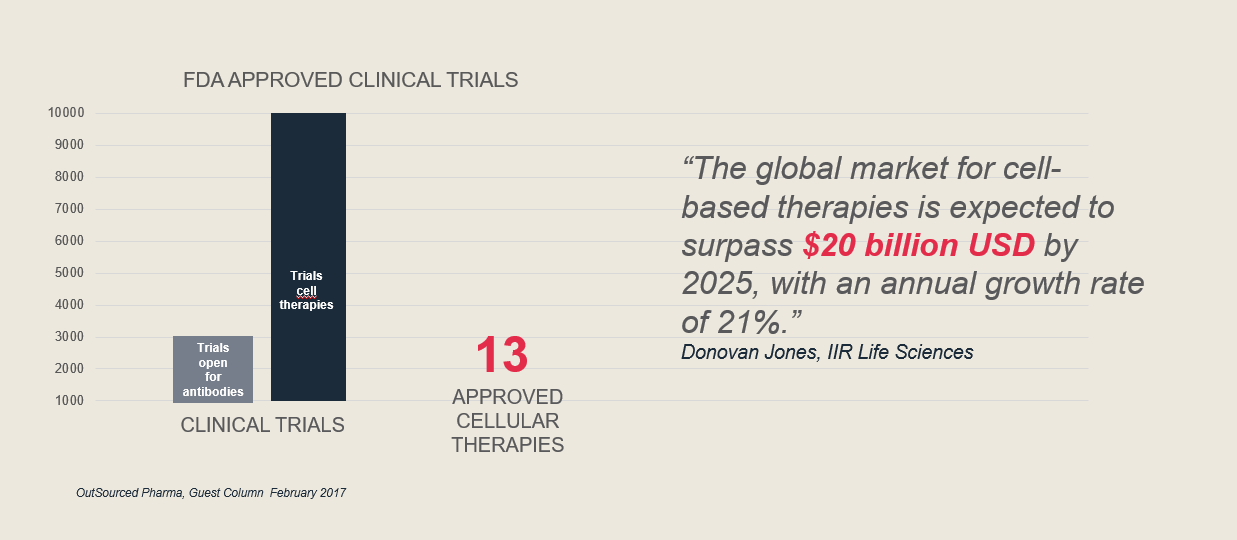
Yet cell and gene therapies can be complex and difficult to work with. To start, getting cell therapies first and fast to market is everybody’s goal in this segment, with competition at a high level.
This means R&D results also need to be delivered faster and more efficiently to get a quick return on investment. In addition, the transition from research to clinical manufacturing needs to be as quick, efficient and smooth as possible.
In this TechTalk article, we will introduce the complexity of CGT and share a case story of a transitional journey from research to early clinical manufacturing in cell-based therapies. Read about the challenges we faced and our reflexions along the way to help you succeed in this evolution now and in the future.
Cell and gene therapies compared to traditional large scale biomanufacturing
To better understand the complexity of CGT, it may be helpful to compare it to something with decades of research and refinement: traditional large scale biomanufacturing.
The main differences are:
- Level of complexity and scale. Unlike traditional biomanufacturing, cell and gene therapies are extremely complex while also being highly variable and on a small (lab) scale.
- Technology and equipment. What is currently available for CGT is typically not developed with GMP in mind.
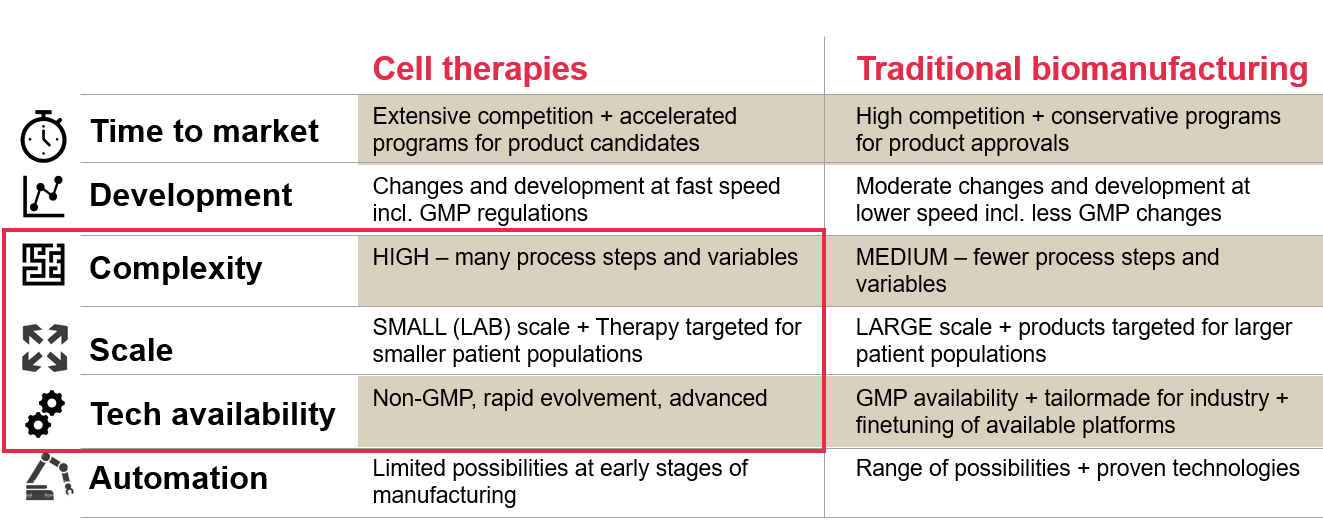
However, it is important to note that processes and equipment for CGTs are developing and advancing exponentially. What you see in the catalogue today may be completely different in just 6 months.
A cell therapy case story from Novo Nordisk
To explain the challenges further, this Novo Nordisk facility in Fremont, California was built/refurbished and established with in-house clinical manufacturing capacity. It will produce cell-based and allogenic therapies that have the potential to provide novel treatments for chronic diseases like Diabetes and Parkinson’s Disease.

This facility is particularly interesting because it offers a concrete example of the journey from research to early clinical manufacturing in cell therapy. Using this as a starting point, we will illustrate certain challenges and finally explain our workable solutions.
Challenge 1: Everything is new in the cell therapy journey
Alongside urgency and competition in the sector, taking any type of cell therapy from research to early clinical manufacturing is a new journey each time, and has never been done before with the same cell therapy. This means:
a) For most of the journey, the technology is brand new.
b) The process, from beginning to end, is often unknown - or at least immature when establishing the manufacturing platform and premises.
Alongside this novelty, variation is an integral part of CGT. In an ideal situation, it would have fixed process parameters, e.g. that the input is the starting material and the process and output is the final product. But because the starting material and the final product (which are cells) are “alive” in CGT, the input and output will always be variable.
Ultimately, there is no key list or template for taking CGT projects from research to early commercial at full GMP. Unlike many other manufacturing settings that have proven platforms and equipment with familiar technology, there are many unknowns and variables in this evolving CGT area.
Challenge 2: The process for CGT is immature - impacting process and facility design
Another issue we face is general process immatureness. For process design, this means:
- An undefined number of final process steps
- Unknown variables
- Undefined set-points
Consequently, it is difficult to conclude which final process equipment you will need.
In addition, the most suitable equipment for the job is not necessarily yet available or developed. This can cause a mismatch between facility concept and process design, ultimately leading to sub-optimal solutions.
For example, in CGT, it is difficult to define the exact use of standard equipment like incubators in the process early on, such as how frequently the material should go in and out, and how much incubator capacity you will eventually need. But if you tailor-make the facility without this information and clarification, you risk a lack of space in the final layout or a lack of flexibility in the (unknown) future.
Challenge 3: Transitioning from R&D to GMP clinical manufacturing
Transitioning from research and development (R&D) to clinical manufacturing means shifting from non-GMP to GMP platforms. This is a big step and raises several key challenges, including:
- Different mindsets – for R&D the core goal is to keep the cells alive. For GMP, it is vital to take contamination and consistency into account.
- Different language. Gowning in research means ‘putting on a lab coat’. In a GMP cleanroom setting, you need masks, gloves, a hairnet, shoes and a full particle-free gown
Ultimately, “clean” means something different in R&D environments versus a GMP environment. Items washed in a dishwasher are ‘clean’ in R&D. In GMP manufacturing, you need to have everything under control – such as the water, cleaning agents, environment, temperature, airflow, and concentration time.
In addition, new technology is common in R&D and equipment is often only fit for R&D lab-scale purposes – with many parts and creative constructions that are difficult to clean. This setup, such as the one on the left below, is rarely GMP compatible.
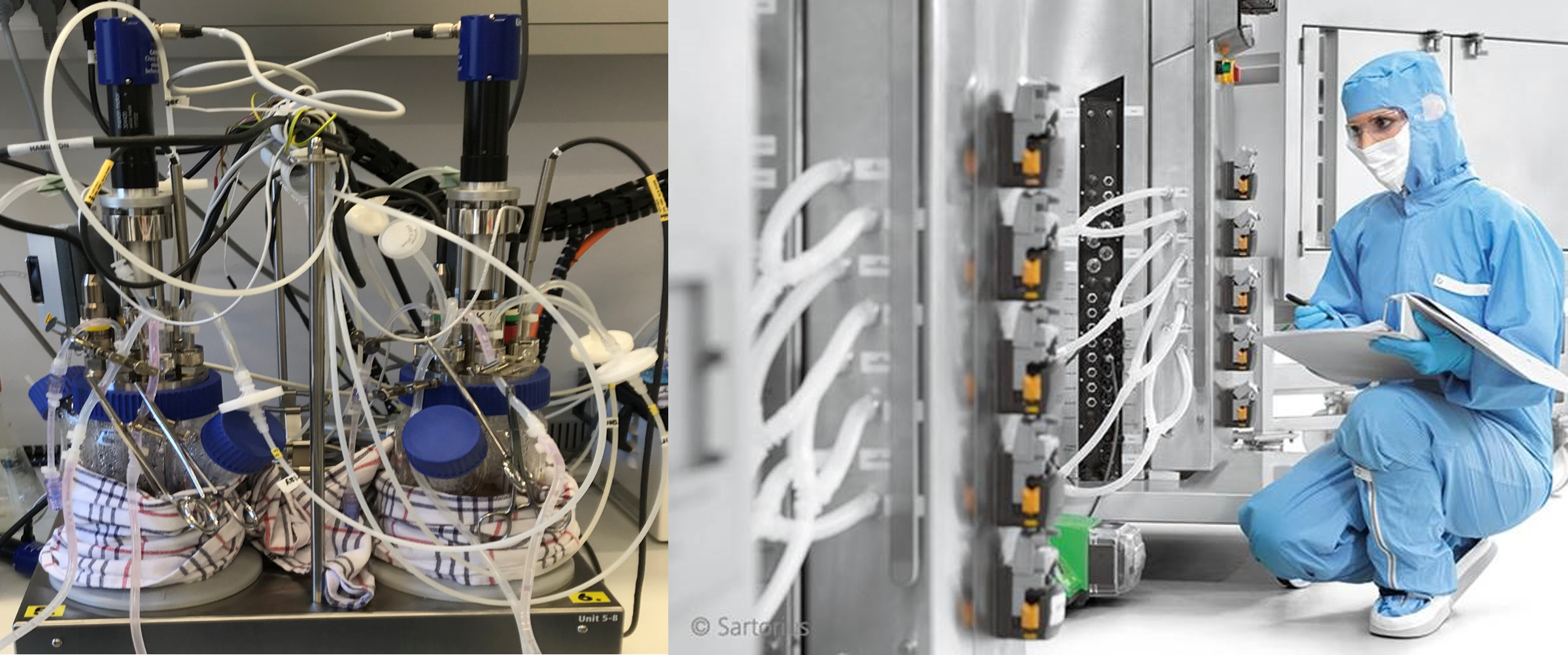
Indeed, it takes a huge amount of effort to identify suitable, GMP compliant equipment – whether it is off the shelf or customized to fit into a GMP setting. Because this is time-consuming, we often end up with solutions somewhere in-between.
Addressing challenge 1: Everything is novel in the cell and gene therapy journey
With all the mentioned challenges in mind, how do we move forward towards success?
When starting this journey in the case mentioned above, first we had to break away from tradition. There’s no time in CGT to wait for a fixed process in order to design a manufacturing facility. Consequently, we detached process and equipment from facility and utility as much as possible. This helped us focus and fast-track the project, with the process developed in parallel with design and construction.
Second, we found it beneficial to focus on both technology and mindset. This means:
- Defining our own template(s)
- Developing a manufacturing facility design concept that is highly independent of process research and adaptable for later changes + future flexibility
- Having the courage to make bold decisions and ensure management will back them up, as not everything is defined and crystal clear from the start
Addressing challenge 2: The process for CGT is immature - impacting process and facility design
To address the challenge of process immatureness, it is important to a) accept it and b) see it as an opportunity for flexibility.
In this case, our initial thinking was very ambitious. We envisioned a future concept with automated processes and limited operator impact. This long-term thinking helped to make the concept and overall direction more clear, however, we realised this ambition was not yet achievable in the early transition from research to manufacturing in CGT. Equipment and technology are still limited or not GMP compliant. And most importantly – the process was not mature enough for this approach.
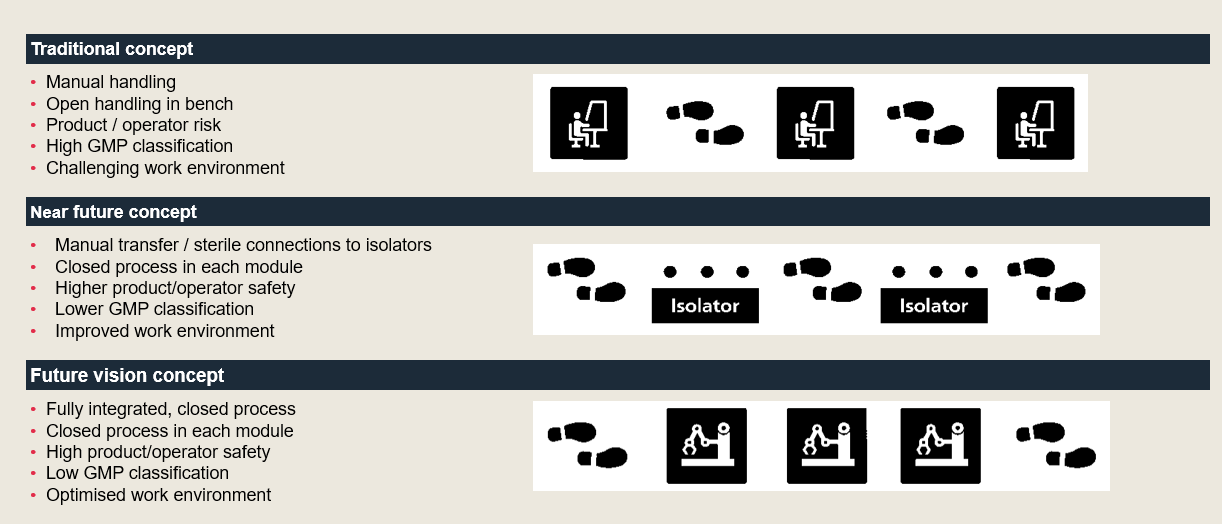
Thus, during design, we scaled back and found it best to use a generic concept that could be adapted later. We started simply with an initial platform based on manual handling in high GMP classified cleanrooms. Then, the long-term strategy is to progress towards automated processes, data handling and eventually lower GMP classification for cleanrooms.
In addition, we used what we already had available – important to get fast(er) to market. In our case, we reused several cleanroom suites in an existing building.
Addressing challenge 3: Transitioning from research to GMP clinical manufacturing
GMP takes time, effort and is often underestimated. Therefore, it is key to adopt a GMP mindset very early on. Instead of choosing the newest model available, it is important to thoroughly explore GMP concepts to understand what will work best for your facility.
In this case, we were aiming for flexibility – and developed a robust segregation strategy using dedicated, segregated, generic GMP suite units (discussed in more detail below). This allowed us to take various cell therapies into account for current and future needs.
We also used a GMP risk-based approach - a powerful tool for getting fast to market, reducing validation time and thereby overall project execution time. To do this, we conducted pre-assessments and pre-tests, thought ahead to address potential issues, and familiarised ourselves with equipment and the process.
Generic and adaptable GMP suites
For the GMP suites, we began by defining a generic, simple concept to serve key processes. These were flexible and adaptable to take future process and process equipment changes into account
To illustrate further, the overall facility concept was like a puzzle, with the generic GMP suite as the main puzzle piece.
- We had two GMP suites as a starting point at the existing facility.
- We built on this and developed a concept around them.
- We added one more GMP suite and supporting functions to run a GMP area (airlocks, storage, gowning area, grade C corridor).
- We finally added another GMP suite - still generic, but for now dedicated to gene editing processing, including the use of viral vectors.
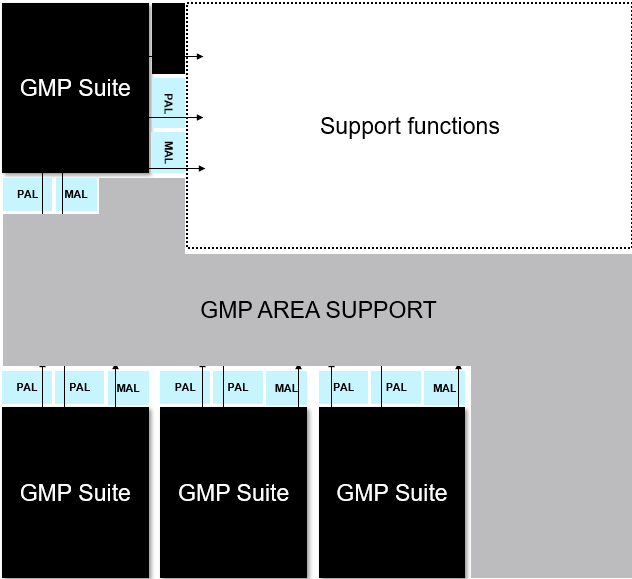
To ensure the areas will be strictly segregated, the GMP suites have suite dedicated airlocks and a dedicated HVAC system, allowing for multiproduct and multiphase process activities. The generic suites can be connected or disconnected from the GMP area, facilitating a high level of flexibility for working with different processes in either research, tech transfer or manufacturing. Classified, not controlled (CNC) support functions will take place outside the GMP area.
Alongside this, most equipment is mobile and plug & play for easy and fast change-over between campaigns. This includes features of easily accessible and changeable utilities, with a simple stainless chase in the upper corner of the walls and ceiling and space for additional utilities if needed at a later stage.
Six key takeaways for your cell and gene therapy journey
Overall, there are six key messages from our case story that may help you in your CGT journey from research to early clinical manufacturing:
- Acknowledge the reality of multiple unknown variables (process immatureness being a huge challenge that impacts everything) and adjust to it
- Think flexibility and adaptability wherever you can – and define what flexibility is specifically in your project
- Implement a GMP mindset from the earliest point of the project – this will ease the transition from research to early clinical and commercial manufacturing
- Use a generic concept design to ease you through the challenge of changes
- Have the courage to take bold decisions – you cannot be on 100% solid ground all the time (since everything is novel) but you can work actively with the risk of this reality
Finally, as an integrated part of our considerations and final output, it was incredibly important to us to
- Work as one team. In this project we built a cross-Atlantic relationship that went beyond traditional borders of disciplines and culture. In addition, involving the right contractors early on, and giving them and the design and qualification teams low-level decision power allowed us to move very quickly.
About the authors:

Henriette Schubert (left): Managing Consultant, Architect MAA from NNE has been engaged in cGMP cell and gene therapy R&D and ATMP facility planning and design since 2005. Focus areas are consulting assignments: creating basis for decision and basis for design and facility design concepts, using a holistic approach to bridge process and cGMP requirements into compliant work operations and facility design. Henriette is a direct consultant to Novo Nordisk for establishing cell therapy manufacturing facilities.
Christina Dragsbaek Ravn (right): Senior Project Manager, M.Sc.Chem.Eng. from Novo Nordisk has worked as project contributor and project manager within the pharma industry since 2001. Focus areas are process design, GMP compliance, qualification, implementation of new processes and facilities, optimization and up-scaling. In her role as Project Manager for an ATMP project, Christina focuses on linking R&D with GMP manufacturing culture. Christina has a lead role in establishing cell therapy manufacturing facilities for Novo Nordisk.