When it comes to assuring product sterility, you can’t take shortcuts – or can you? Single-use systems could be the key to speeding up change-over of fill finish production lines without compromising sterility.
After a series of complex processes, parenteral fill finish is the final manufacturing stage of sterile dosage drugs. Since they are administered intravenously there are critical safety requirements and risks, and to ensure the product is sterile global regulatory authorities have stringent guidelines on the implementation of robust operational and quality systems.
In the case of aseptic drug products, the requirements for assuring sterility are particularly important. Since the final dosage form is assembled from components that are sterilized separately, the individual sterilization and aseptic processes must be robust, repeatable and fault-tolerant. This is particularly crucial because we cannot test the sterility of the finished product.
Improving preparation time
In traditional aseptic manufacturing processes, making sure sterility is at a mandatory level requires a lot of time, labour and resources. To give you an example, a typical serum-filling operation that takes 8 to 16 hours to complete may take 48 to 72 hours to prepare.
This is because a conventional stainless-steel product pathway requires intensive sanitization and sterilization processes to establish and assure product contact sterility. Since many of these steps are manual they must be performed by specially trained and qualified technicians to achieve asepsis.
Why improve changeover capability?
Single-use systems (SUS) on the other hand effectively eliminate many of these critical steps and further reduce change-over time by simplifying end-of-batch breakdown and decontamination operations. SUS, forged and refined during the last 25 years of biotechnology development, has significantly improved changeover capacity by eliminating time-consuming cleaning and preparation steps. SUS has now expanded into fill finish operations and is making inroads into the traditionally conservative field of aseptic production.
What are the business incentives for using single use systems?
From a business perspective, the evolution of this technology centres around four emerging themes in pharmaceuticals:
- A move to personalized healthcare - involving smaller batch sizes and more frequent product change-overs
- An increasing variety of molecular entities that need flexible manufacturing capabilities
- Co-location of large and small molecule fill finish operations, which minimizes capital investment
- Cost pressures from society and government - driving our industry to reduce the cost of goods sold
When comparing the pharmaceutical industry with other business sectors production lead time and finished goods inventory are significantly high, whereas overall equipment effectiveness (OEE) is consistently low.
Why is this? Certainly, cGMP and regulatory requirements are the main cause, but the constraints imposed by traditional fill finish manufacturing methods influence this as well. There is a clearly a need to increase operational capacity and reduce operating costs if we wish to fulfil the economic and medical needs of patients worldwide.
Should we stick with stainless steel, or try single-use?
The table below highlights the set-up and breakdown durations of a traditional stainless steel aseptic pathway compared with a disposable SUS solution. The time estimates are based on a state-of-the-art filling isolator infrastructure that involves the set up of open isolator product pathways followed by vaporized H2O2 sanitization, needed to establish the ISO class 5 aseptic environment.
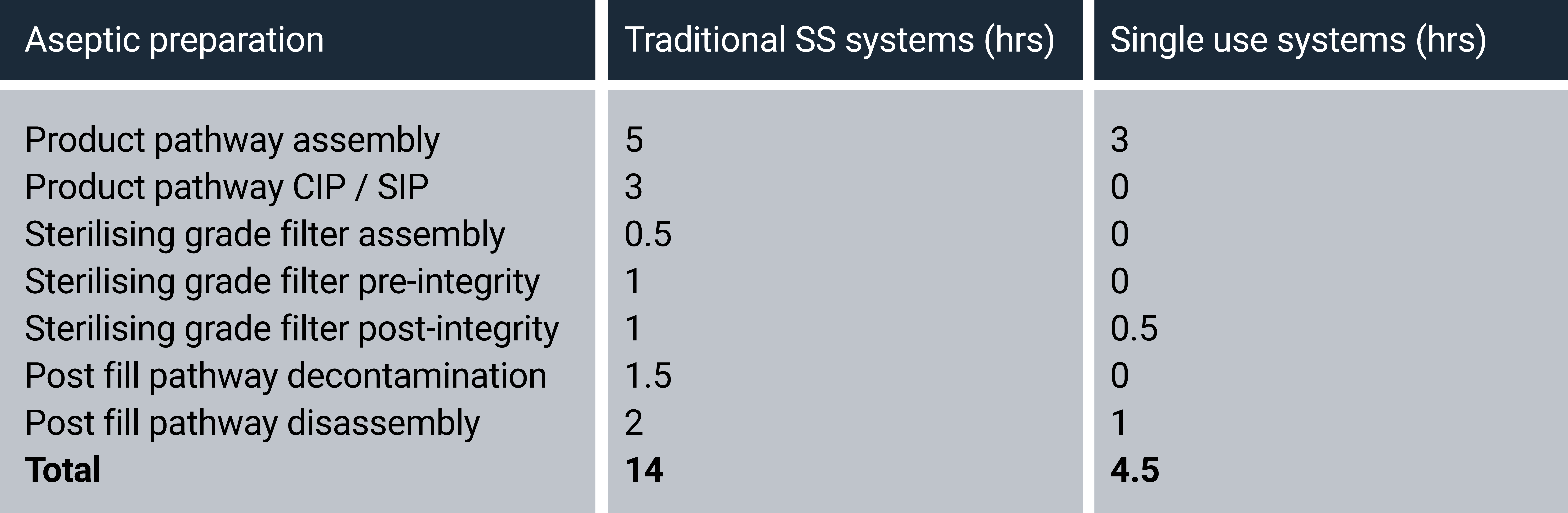
This comparison demonstrates that a SUS system can be installed and made operational in around a third of the time required for a traditional stainless steel product pathway. Beyond the time saved by SUS technology alone, using single use systems can also mean streamlining additional processes due to the elimination of cleaning and sterilization (CIP/SIP).
Through many years of development and utilisation for bulk biologic manufacturing, single-use systems have proven to be beneficial for the pharma manufacturing industry. Advancements in aseptic drug product manufacturing involving isolators and restricted-access barrier systems (RABS) have created robust sterile manufacturing environments that effectively separate human operators from the fill finish process. Leveraging the capabilities of state-of-the-art aseptic filling environments with SUS product pathways opens the door to significant improvements in operational time efficiency, improved patient safety and overall economic value for consumers.


