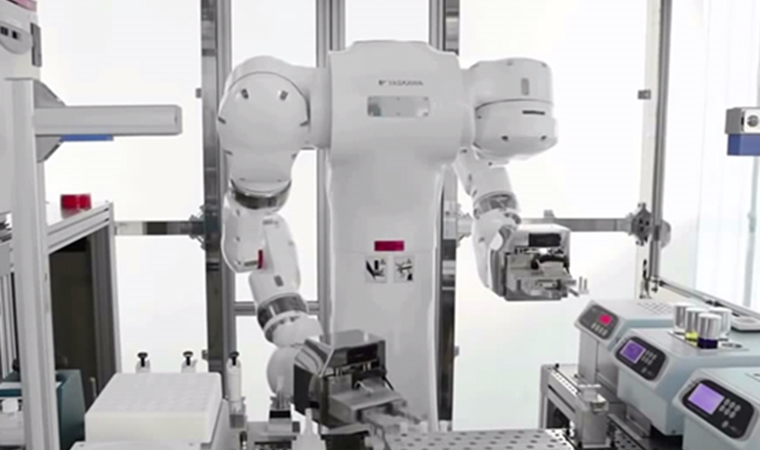
The pharmaceutical industry has been slow to adopt robotic technology compared with its manufacturing peers. But with the FDA encouraging the use of innovative technologies, it’s only a matter of time before the use of robotics in biotech goes from first-mover to standard.
Robotic technology has developed dramatically over the last decade and many industries have embraced it, from the automotive world to electronics. Finally, the healthcare industry is following suit and starting to take advantage of robotics. But how will this affect the world of biotech?
Biotech, meet robots
Traditionally one of the first production steps in biotech is when the chemist handles a cell culture. This cell culture process requires a high level of sterility, so the chemist wears gloves and goggles and works under a laminar flow hood to prevent contamination. It is a critical production step with a high risk of cell contamination (which unfortunately does happen from time to time) and should be repeated the same way every time. It therefore requires a high level of accuracy from the chemist.
Why would robots help in this situation? Because with people, all of these parameters – repeatability, accuracy, sterility – are at risk. Human error is something we unfortunately cannot avoid. Robots, on the other hand, execute with a very high level of accuracy and duplicate that accuracy repeatedly.
A robot can also work in a fully closed and sterile environment, and now several robot vendors have approved robots that can be used in class A clean rooms. These robots are even suitable for decontamination processes in vaporized hydrogen peroxide (VHP) environments.
Beyond the benefits of repeatability, accuracy and sterility, robots:
- Do not need gowning and all of the related processes around wash and transport of gowning
- Do not have to read standard operating procedures (SOPs) or receive regular training
- Can operate under poor working conditions and in strange positions without compromising health, safety or environmental regulations
- Can switch between several programs, offering a high degree of flexibility
One of the final steps in the biotech process is often a "bulk flask filling,". This again has high requirements for repeatability, accuracy and sterility. Robots can also execute this process, tapping into the benefits listed above, and be equipped with cameras to document significant production steps where needed.
But perhaps the greatest advantage of robots is that chemists are released from monotonous work and can instead concentrate on more value-adding tasks in the cell culture process.
Logistical advantages of using robotics in pharma
Beyond biotech, robotic solutions contribute to general manufacturing goals. These include:
- Their ability to take samples from the process and transport them to quality control, until process analytical technology (PAT) instruments take over and perform the analysis online and provide feedback to the control systems
- Safe lifting and emptying of large bags. In oral solid dosage (OSD) facilities, handling powder bags generates a great deal of dust and often, operators manage this in protective gowning. Instead, the robot could take over the heavy lifting and remove the exposure of dust to the operators
- Load and unload of materials to machines. Finished pharma facilities have several load/unload and transport of materials to and from production equipment. The production could be made continuous from filling to inspection, by allowing a robot to unload the filling machine and automatically transport to inspection or freeze-drying
Why should we embrace robotics as an industry?
Robotic technology is widely used in other industries, some of which are comparable to pharma. It is even used by the automotive and electronics industry where cost is vital, requirements for cleanliness are higher than pharma and where the need for agility and flexibility is key to survival.
Furthermore, the US Food and Drug Administration (FDA) encourages adoption of innovative approaches to pharmaceutical product design and manufacturing. In fact, the FDA has established an Emerging Technology Team (ETT) so industry representatives can meet with ETT members to discuss, identify and resolve potential concerns regarding the development and implementation of a novel technology prior to filing a regulatory submission.
The technology is there, peers have used it for decades and the regulatory authorities are encouraging us in the pharma world to embrace robotics. It’s only a matter of time before pharma facilities that embrace robotics go from first-movers to standard operators.