The rise of aseptic technology, and the manufacturing and regulatory challenges that come with it, will inevitably impact project execution. Find out what lies ahead for fill and finish projects in the conflict of market requirements.
In this article, “Aseptic technology on the rise”, Hartmut Schaz provides an interesting account of the future of the pharmaceutical market and the technological and regulatory changes driven by the rise of aseptic processing. In this article, I will pick up where Schaz left off, and look into how the supplier industry, such as machinery and packaging material manufacturers, is expected to react to the new market situation.
How will market development affect projects in the future? In what direction will machine delivery times, building construction periods and overall project duration develop? And how will project costs develop?
The expectation is that time and costs in fill and finish projects will be influenced negatively. But in fact, the opposite seems to be the truth.
How are equipment suppliers adapting to the rise of aseptic technology?
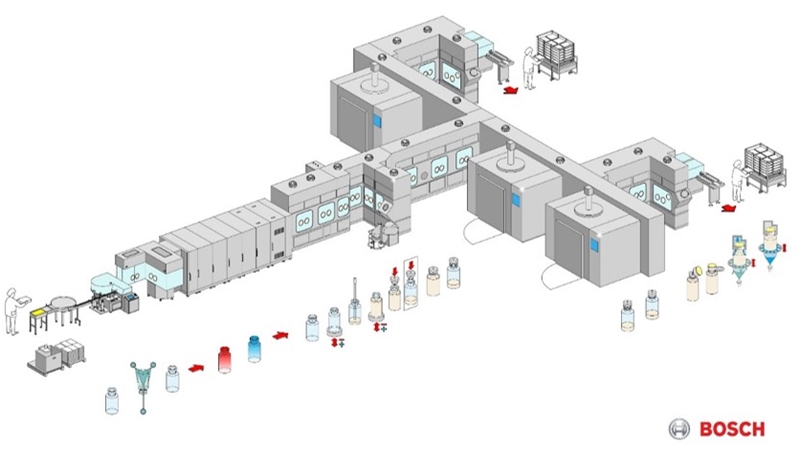
Equipment suppliers have been observing the market situation for a while and have adjusted their equipment to serve these new requirements. This can be seen with modularity and the level of automation, such as introducing more robotics solutions.
Well-known filling line manufacturers such as Bausch + Ströbel or Groninger have been offering modular lines in isolator technology for some time, and use a variety of modules to maintain the required flexibility with regards to the use of different primary packaging materials. Particularly impressive are the filling lines or filling cells from newcomers such as Vanrx or Pharma Integration, whose systems can almost be used without gloves.

Some suppliers of primary packaging material are already able to deliver almost their entire portfolio as RTU (ready-to-use). It is often available both as a bulk (single container) and in a nested arrangement versions. These primary packaging materials are already pre-inspected for damages or other defects. Due to standardized nest sizes, format changes are almost redundant.
Early alliances between machinery and packaging manufacturers mean that both parties have made adaptations to their respective products to match each other’s. Coordination and standardization thus clears the way for a fill and finish technology that can be performed by robots only. Thus, as Schaz pointed out, people ( regarded as the greatest risk of contamination) no longer need to perform any critical production process that might result in a viable or non-viable contamination of the product. Instead, they can focus their attention on monitoring and control tasks.
How does this affect delivery times, costs and quality of the equipment?
The technical design of modules will have a high degree of standardization, requiring much less effort in the design, engineering and implementation. Also, the consistent design of the modules will drastically reduce both costs and delivery times.
A customized filling line has an average delivery time of 20 months. In comparison, suppliers claim they need only 8-10 months to deliver the modular units. Factory acceptance tests and qualification pre-tests are also standardized, therefore need significantly less times for tests.
What does this mean for the overall project plan?
In projects with tailor-made process machines, early development of building planning very often fails due to missing information from the equipment suppliers. They simply do not have the technical information, such as utility connections, layout and dimensions, at this early stage. This leads to delays in both building design and building execution.
Again, the desired modularity and the associated standardization can remedy this situation, providing a much smaller number of interfaces with less complexity to the building and utilities. The information required for the building can be made available almost immediately after purchase order, resulting in shorter delivery times.
With early knowledge of machines and their technical details, commissioning and the associated test phase can be approximately halved. Where several months have been needed so far, it is now possible – even for more complex machines – to complete the preparation work for the qualification within weeks.
It would be anticipated that time and costs in fill and finish projects will be influenced negatively. But in fact, the opposite seems to be the truth."
Modular standardization also ensures a high degree of planning security during the qualification phase. The procedures for the execution of qualification are tested several times in the same modules and quality assurance has the opportunity to check and accept the qualification plans and qualification documents at a very early stage.
If the responsibility for the complete execution of the installation and operational qualification remains exclusively with the machine manufacturer – and the customer is only limited to monitoring and control – the qualification of the process machines has almost no time risk and accordingly no cost risk, like ad hoc shortage of qualification resources.
Depending on the type and scope of the process, we need to consider that the investment costs for a fill and finish project calculates 35-45% of the TIC for process machines including their qualification. Here, the opportunities arising through modularity inevitably leads to significant cost savings. This is certainly not caused by lower equipment costs itself, but rather by reducing the follow-up costs for setup, commissioning, qualification and the involved time savings.
A significant factor should be mentioned here: The operating costs of a modular, highly flexible fill and finish production with exclusively RTU packaging (regardless of whether nested or bulk) will obviously be much higher than the operating costs of a conventional production line. Depending on the production quantities, batch sizes and products, this cost difference can vary a lot, but rough calculations lead to a factor of about 1:10 up to 1:20 or more, to the disadvantage of RTU packaging. Since production capacity within the RTU industry is constantly increasing, this ratio is most likely to decline significantly in the future.
However, when considering the higher costs of this extremely high production quality to the horrendous values of a single dose of biotech product, this seems quite justifiable.
Image provided by Vanrx Pharmasystems.