Written by Roxanne Batty. Expert input from Rojan Demirtas, Consultant in Compliance Consulting and Karin Hedebo Wassard, Senior Specialist of Process Operations at NNE.
Cell therapy is a groundbreaking approach to handling diseases that were previously untreatable. The cell culture process is a crucial step and needs to be carefully conducted to avoid contamination. Yet manual handling is still common. Could closing and automating the process be the right choice?
The pharma industry and its regulatory authorities are shifting towards closed, automated systems in many drug manufacturing areas. The clear advantage is that these systems tend to be safer; there is less risk of contamination which means less risk of losing the batch or harming the patient. There is also evidence that embracing this technology can significantly reduce space, energy consumption, staff numbers, and approval time.
Many cell therapies are produced with only one batch per patient. The consequences of losing or contaminating a batch, therefore, can directly impact a person's life. Closing and automating the process can be a great way to alleviate this risk. However, these systems are also a large investment and not necessarily suitable for all product types.
Cell culture production is the stage where patient cells at multiplied under controlled conditions. To give you a clearer idea of whether closed, automated technology is the right choice, this article outlines three approaches to this stage and the associated operational and environmental costs.
Cell therapy manufacturing - a quick introduction
Cell therapy manufacturing, in simple terms, starts and ends with the patient. It starts with the collection of cells from a patient in a medical facility and ends with the administration of the drug to that same patient. But between these two points many complex processes take place.
Equipment systems used in the various steps of cell therapy manufacturing may include instruments for cell collection, cell isolation or selection, cell expansion, cell washing and volume reduction, cell storage, and finally cell therapy transportation.
These steps can vary immensely based on the manufacturing model, e.g. autologous, meaning one batch per patient vs. allogeneic, meaning a single source of cells is used for many patients. They are also affected by cell type and the intended purpose of the therapy.
In addition, cells are “living” entities that are highly sensitive to even the simplest manipulations. Thus, cell therapy manufacturing equipment and the potential for automation to reduce error are hugely important.
Open and manual cell culture production
Open, manual processes are currently the norm for autologous cell therapy products and are the starting point for most new therapies during research and development. Culturing patients’ cells with open culture vessels in the early phases of research and development (R&D) is the most established and traditional method. Because of this, equipment is low-cost and safety protocols are in place.
The downsides of using open, manual processes in a cGMP production setup are the risks of contamination, operator exposure, and human error.
For example, in R&D, common steps such as volume reduction and buffer exchange are typically performed manually using a benchtop centrifuge followed by repeated manual pipetting to achieve a cell suspension. This same process can be performed in a Class B room biosafety cabinet in a GMP manufacturing facility. However, the frequent opening and closing of vessel lids in this approach increases the risk of contamination.
[Read more from Li, et al. 2021 “Advances in automated cell washing and concentration”].
Thus, Standard Operating Procedures (SOPs) are crucial to avoid contamination and to separate batches for different patients, alongside skilled operators experienced with cleanrooms and biosafety cabinets to perform and monitor the process.
In addition, open manual operations conducted in class B cleanrooms under class A processing areas have significant HVAC running costs. So, despite the low initial investment on equipment, this approach requires more space, full-time employees, and energy. It is also higher risk and costs more in the long run.
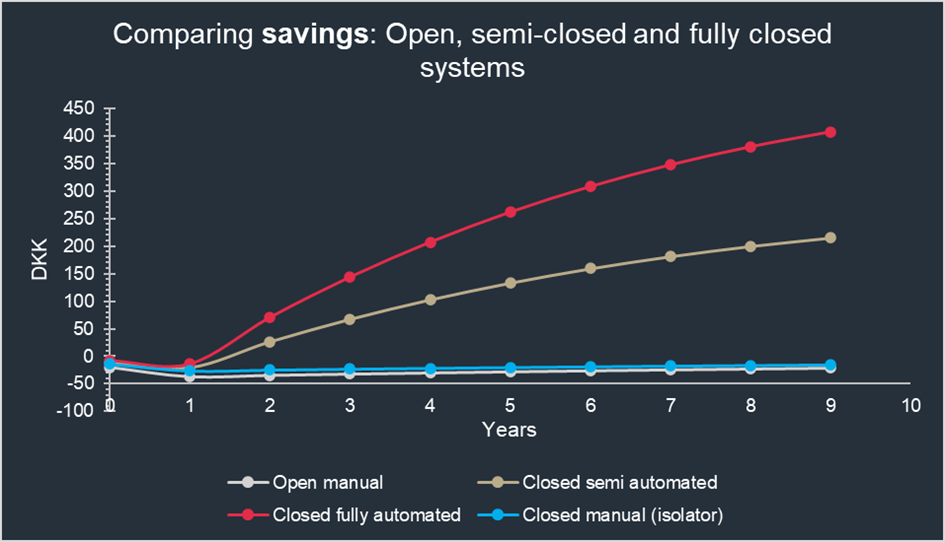
Figure 1: Comparison of different cell culture processing approaches. In the longer run, closed fully automated operations will result in significant savings due to the facility footprint, HVAC cost, and FTE cost. The scenario calculations are based on processing 2000 Batches of autologous product per year, and are partially inspired by the scenarios in James, D., 2017. How short-term gain can lead to long-term pain. Cell & Gene Therapy Insights and typical data from suppliers, as well as internal NNE cost data. Read more on the data here.
If you choose to start your cell therapy production using a manual approach, it is important to consider the evolution of your production process and changes in commercial scale over time. It is likely you will need to incorporate closed, automated processes at a later stage, and it is easier to do so if you have included this consideration early on. Utilizing equipment that lends itself to both automation and potential scale-up in a closed manner from the development phase onwards will reduce the automation “leap” when the time comes.
Semi-closed and automated cell culture production
If the investment of fully automating your process is currently too high, an interim step could be to partially close and automate cell culture production. By doing so, you still benefit from reduced long-term costs and reduced risk (see the graph below for more details).
Indeed, this approach may be the best and only option. Depending on your process and the therapy you are producing, you may have some process steps that are not appropriate for a closed automated process, such as vial handling, transfer from vials, incubator use, and small-scale filling.
Semi-automated may also be the best solution depending on the cells you want to culture and the delicate balance of temperature, humidity, media, and buffer they need. In some cases, cells won’t grow well in closed, fully automated systems. In these cases, utilizing a hybrid approach with multiple pieces of single process equipment, where product transfer is accommodated by welding, could be the best option.
Scalability is also an important consideration when deciding on the right approach for cell therapy manufacturing. If you are producing a lot of cells, there are currently only a few available large-scale options when the process is not suitable for suspension. Although there are a few adherent options available at a larger scale, companies often settle on a semi-closed approach that can handle the seeding process from vial to seed for larger bioreactors using well-established technology. Closed processes are not yet fully available for large-scale cell manufacturing.
Fully closed and automated cell culture production
With the above considerations in mind, technology is now available for manufacturers to establish, grow, and maintain cell cultures in an automated, closed system for smaller batch sizes. This is ideal for autologous products. It also has the obvious advantage of replacing manual operations and increasing precision while lowering risk, costs, and the amount of repetitive manual work.
Moving to a fully automated system has the added benefit of opening the door to better manufacturing intelligence. Collecting the right data and using this information well can increase reliability, repeatability, and speed up batch approval (which can be long for cell therapies). For example, “Release by exception” – where only exceptions from pre-established targets need to be reviewed by quality teams – can be a huge advantage for companies that need to quickly get their medicine to patients. This is especially important for patients with life-threatening conditions who have an urgent need for their therapy.
Overall, compared to classical aseptic cell manufacturing, automating cell culture processes has many benefits.
Lower cleanroom requirements and reduced space, which:
- Reduces cost in facility establishment
- Reduces the operational cost of a facility
- Reduces the number of operators
Increased product quality through process closure and automation, which:
- Decreases contamination risk
- Reduces operator-to-operator variability and handling errors
- Standardization of complex procedures in closed systems for product and operator safety
- Reproducibility of GMP-compliant cell products
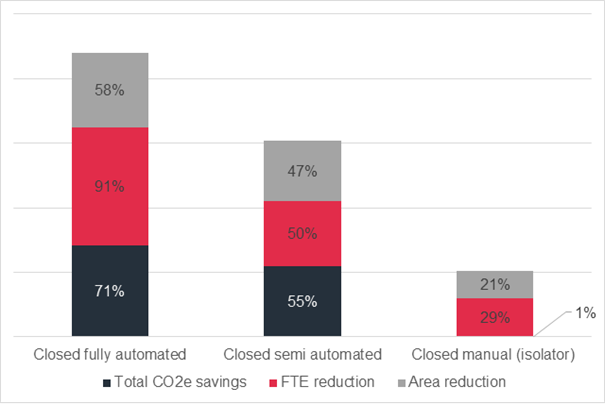
Figure 2: Illustration of estimated savings in total CO2e, FTE and Area compared to open manual process. Read more on the data here.
Conclusion
It is perfectly fine if your setup is not yet mature enough for automated, fully closed processes. It is also perfectly fine if the therapy you are producing works better in a semi-automated system. What is important, however, is to consider the long-term evolution of your operations and consider potentially relevant automated systems in the early stages of process development, to allow for easier transfer once the process is mature.
Due to the risks of manual operations, automated, closed systems are a matter of when not if. In the far future, there is a real possibility that we will automate the entire process with zero operator intervention. For now, depending on your company goals, the benefits of partially or fully closing and automating your autologous cell culture production are evident.
If you’d like to learn more about the advantages and disadvantages of the technology available and what could be the right choice for your unique setup, please contact us below.